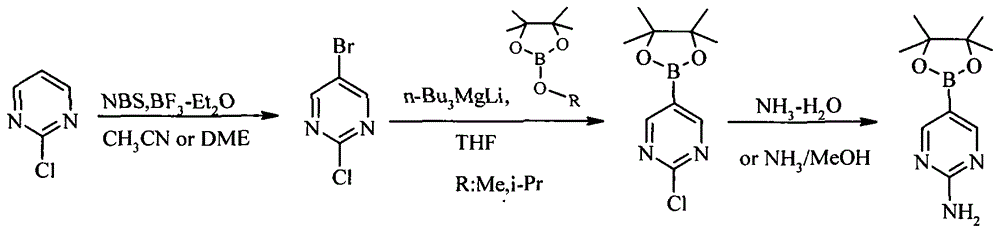
A method of preparing 2-aminopyrimidine-5-boronic acid pinacol ester
An aminopyrimidine and alcohol ester technology, which is applied in the field of pharmaceutical intermediate synthesis, can solve the problems of large amount of coupling catalyst, heavy metal residues in the product, and the need for ultra-low temperature reaction, and achieves the effect of mild synthesis process conditions and avoiding ultra-low temperature reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A method for preparing 2-aminopyrimidine-5-boronic acid pinacol ester, comprising the following steps:
[0017] Add 11.5g (0.1mol) of 2-chloropyrimidine, 21.3g (0.12mol) of brominating reagent NBS and 80mL of acetonitrile into the reaction flask, and add a catalytic amount of BF at room temperature 3 -Et 2 O 2.8g (0.02mol), after adding, be warming up to reflux reaction 5-8 hour, after TLC detects that reaction is finished, cool down, after filtering out insoluble solid, after depressurizing distillation solvent, add ethyl acetate to dissolve, dropwise add 45mL saturated Na 2 CO 3 The solution was washed, the organic layer was separated, and 60 mL of ethyl acetate was added to the aqueous layer. After layering again, the organic layers were combined, washed with saturated saline, and spin-dried to obtain 14.1 g of light yellow 2-chloro-5-bromopyrimidine solid, which was directly used in the next reaction. 1 H-NMR (400MHz, CDCl3): 8.70 (s, 2H).
[0018] Under the pr...
Embodiment 2
[0021] A method for preparing 2-aminopyrimidine-5-boronic acid pinacol ester, comprising the following steps:
[0022] Add 11.5g (0.1mol) of 2-chloropyrimidine, 21.3g (0.12mol) of brominating reagent NBS and 80mL of ethylene glycol dimethyl ether into the reaction flask, and add a catalytic amount of BF at room temperature 3 -Et 2 O 4.2g (0.03mol), after adding, be warming up to reflux reaction 5-8 hour, after TLC detects that reaction is finished, cool down, after filtering out insoluble solid, after depressurizing distillation solvent, add ethyl acetate to dissolve, dropwise add 45mL saturated Na 2 CO 3 The solution was washed, the organic layer was separated, and 60 mL of ethyl acetate was added to the aqueous layer. After layering again, the organic layers were combined, washed with saturated saline, and spin-dried to obtain 13.2 g of light yellow 2-chloro-5-bromopyrimidine solid, which was directly used in the next reaction. 1 H-NMR (400MHz, CDCl3): 8.70 (s, 2H).
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com