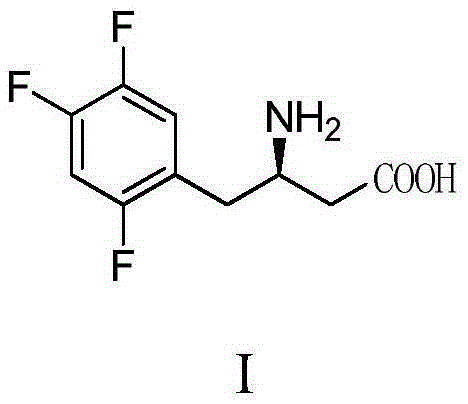
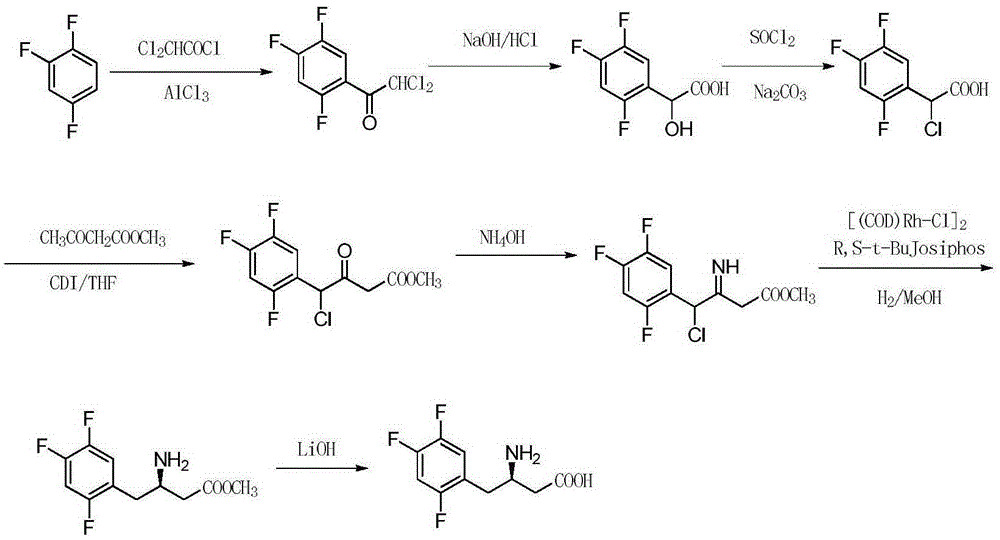
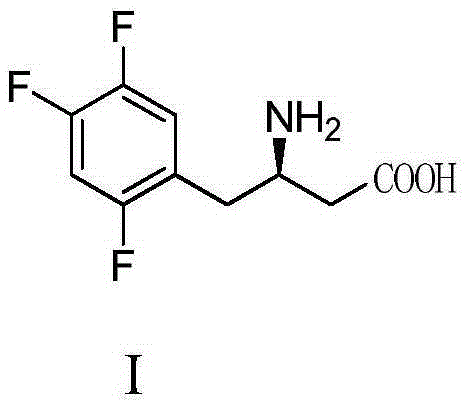
Synthesizing method of sitagliptin key intermediate
A synthesis method and technology of sitagliptin, applied in the field of medicine, can solve the problems of increasing protection and deprotection steps, expensive use, etc., and achieve the effects of easy control, mild synthesis process conditions, and improved optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of α,α-dichloro-2,4,5-trifluoroacetophenone:
[0029] Add 14.7g (110mmol) of aluminum trichloride and 13.2g (100mmol) of 1,2,4-trifluorobenzene in a 250ml three-necked flask, and add 16.2g (120mmol) of dichloroacetyl chloride dropwise under stirring, and the reaction temperature is controlled at Between 40°C and 45°C; after the reaction is completed, lower the reaction solution to room temperature, slowly add 200ml of ice water to remove residual aluminum trichloride, stir the reaction solution and let it stand for stratification; the water phase is extracted three times with dichloromethane and combined Organic layer, the organic layer was washed with water and saturated sodium bicarbonate solution, then dried over anhydrous magnesium sulfate, dichloromethane was evaporated under normal pressure to obtain α,α-dichloro-2,4,5-trifluoroacetophenone 23.6g, purity 98%, yield 95.2%. 1 HNMR (300MHz, CDCl 3 )6.73(m,1H),6.92(s,1H),7.45(m,1H). MS:m / z244(M+H) + . ...
Embodiment 2
[0031] Preparation of 2-hydroxy-2-(2,4,5-trifluorophenyl)acetic acid:
[0032] Add 24.3g (100mmol) of 2,4,5-trifluoro-dichloroacetophenone into a 500ml three-necked flask, slowly add 200ml of 10% aqueous sodium hydroxide solution under stirring at room temperature, and keep the reaction temperature at 0-5°C. After the reaction was completed, the pH was adjusted to 1-3 with 10% dilute hydrochloric acid, and a light yellow solid was precipitated, which was recrystallized from ethyl acetate to obtain 18.2 g of a light yellow solid with a purity of 97% and a yield of 85.6%. 1 HNMR (300MHz, CDCl 3 )3.65(s,1H),5.38(s,1H),6.48(m,1H),6.76(m,1H),10.1(s,1H). MS:m / z207(M+H) + .
Embodiment 3
[0034] Preparation of 2-chloro-2-(2,4,5-trifluorophenyl)acetic acid:
[0035] Add 100ml of anhydrous dichloromethane, 20.6g (100mmol) of 2-hydroxy-2-(2,4,5-trifluorophenyl)acetic acid, and 36.3ml (500mmol) of thionyl chloride into a 250ml three-necked flask under stirring. , reflux reaction for 10 hours, TLC thin layer monitoring to the end of the reaction; after the reaction is completed, add the reaction solution to a 10% sodium carbonate solution cooled with an ice-salt bath, stir for 1 hour, adjust the pH to 1 to 3 with dilute hydrochloric acid, and let the reaction solution stand Layering; the aqueous phase was extracted three times with dichloromethane and the organic layer was combined, the organic layer was dried over anhydrous magnesium sulfate, and the dichloromethane was evaporated under normal pressure to obtain 2-chloro-2-(2,4,5-trifluorophenyl ) acetic acid solid, recrystallized from methanol to obtain 21.7 g of a light yellow body with a purity of 98% and a yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com