Method for preparing polyether-modified silicone oil in presence of MOFs (metal-organic frameworks) supported catalyst
A technology of supported catalyst and polyether-modified silicone oil, applied in chemical recovery and other directions, can solve problems such as no binding, and achieve the effect of reducing synthesis cost and not reducing catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
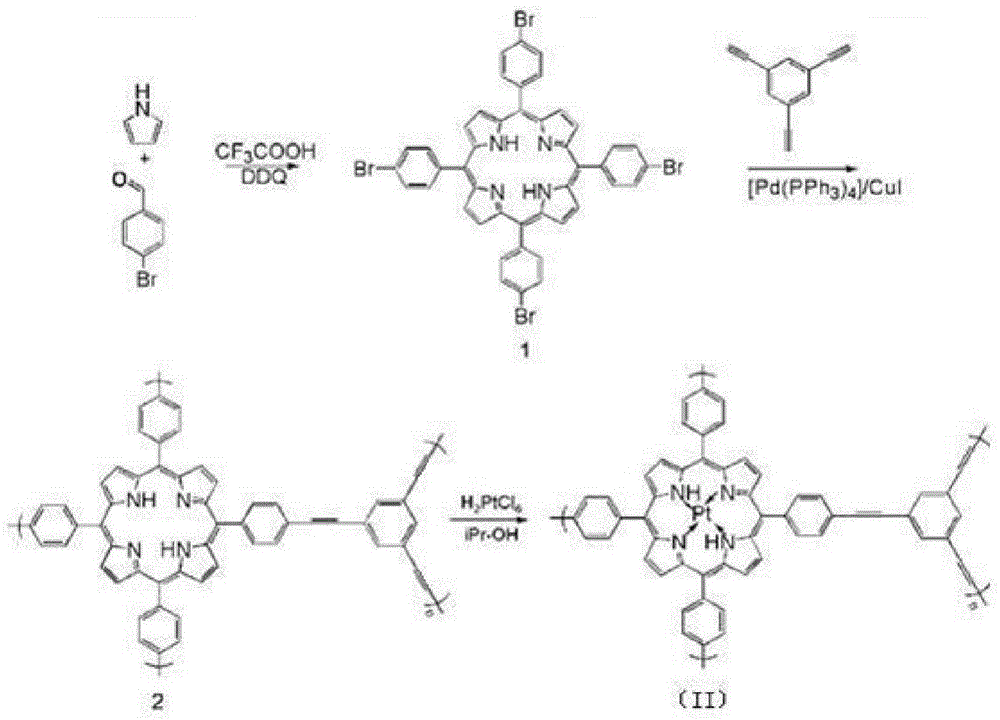
[0049] This embodiment has the synthesis of the MOFs supported catalyst of structural formula (II), and its synthetic route is as follows figure 1 shown.
[0050]
[0051] Its synthetic steps are:
[0052] (1) Under the protection of nitrogen, add 1.3g of pyrrole and 3.2g of p-bromobenzaldehyde into a 2L three-necked flask, add 1.5L of dichloromethane after drying and dehydration at room temperature, and add 3.7mL of trifluoroacetic acid after 10min, Stir at room temperature under nitrogen protection for 1.0h, then add 9g DDQ (dichlorodicyanobenzoquinone), continue to stir for 1.0h, then remove the solvent under reduced pressure, and the crude product is separated using a chromatographic column (stationary phase: silica gel; Mobile phase: petroleum ether / dichloromethane with a volume ratio of 1:1) to obtain organic framework monomer compound 1 with a yield of 30%; mass spectrometry test results: (MALDI-TOF): m / z=926.9, calculated value: 926.9;
[0053] (2) Under nitrogen p...
Embodiment 2
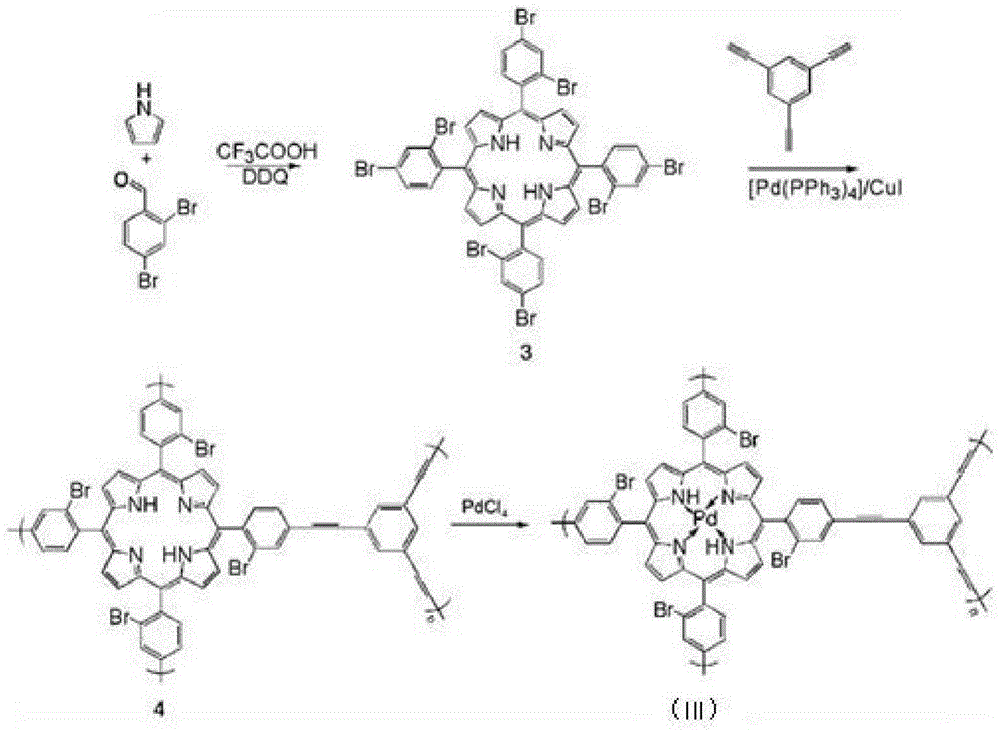
[0056] This embodiment has the synthesis of the MOFs supported catalyst of structural formula (III), and its synthetic route is as follows figure 2 shown.
[0057]
[0058] Its synthetic steps are:
[0059] (1) Under the protection of nitrogen, add 1.3g of pyrrole and 4.7g of 2,4-dibromobenzaldehyde into a 2L three-necked flask, add 1.5L of dichloromethane after drying and dehydration at room temperature, and add 3.7mL of tris Fluoroacetic acid, stirred at room temperature under nitrogen protection for 1.0h, then added 9g DDQ (dichlorodicyanobenzoquinone), continued to stir for 1.0h, then removed the solvent under reduced pressure, and the crude product was separated using a chromatographic column (stationary phase : silica gel; Mobile phase: petroleum ether / dichloromethane with a volume ratio of 1:1) to obtain organic framework monomer compound 3 with a yield of 30%; mass spectrometry test results: (MALDI-TOF): m / z=1245.9, Calculated value: 1245.9;
[0060] (2) Under n...
Embodiment 3
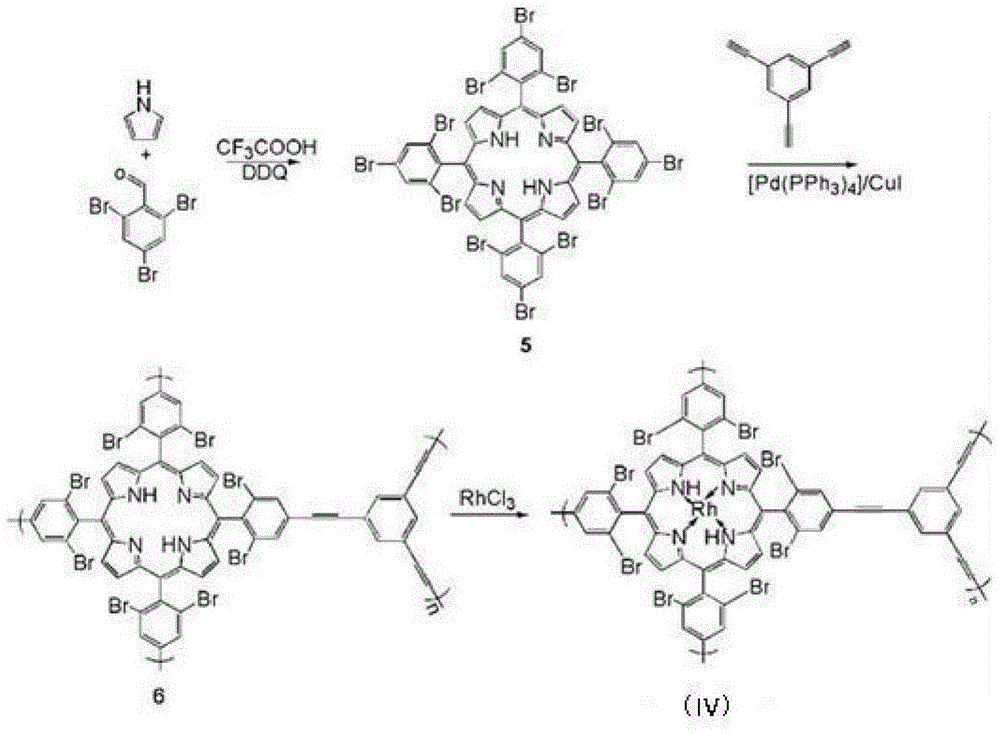
[0063] This embodiment has the synthesis of the MOFs supported catalyst of structural formula (IV), and its synthetic route is as follows image 3 shown.
[0064]
[0065] Its synthetic steps are:
[0066] (1) Under nitrogen protection, add 1.3g of pyrrole and 6.2g of 2,4,6-tribromobenzaldehyde into a 2L three-necked flask, add 1.5L of dichloromethane at room temperature, and add 3.7 mL trifluoroacetic acid, stirred at room temperature under nitrogen protection for 1.0h, then added 9g DDQ (dichlorodicyanobenzoquinone), continued to stir for 1.0h, then removed the solvent under reduced pressure, and the crude product was separated using a chromatographic column ( Stationary phase: silica gel; Mobile phase: petroleum ether / dichloromethane with a volume ratio of 1:1) to obtain organic framework monomer compound 5 with a yield of 30%; mass spectrometry test results: (MALDI-TOF): m / z= 1561.5, calculated value: 1561.5;
[0067] (2) Under nitrogen protection, 8.9g of compound 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com