Imidazopyridine compound
A compound, pyridyl technology, applied in organic chemistry, drug combination, blood diseases, etc., can solve problems such as undisclosed or revealed sGC activators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
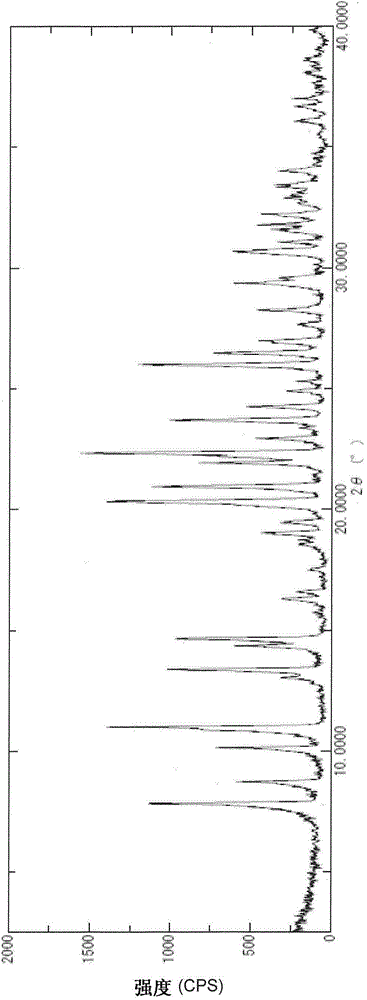
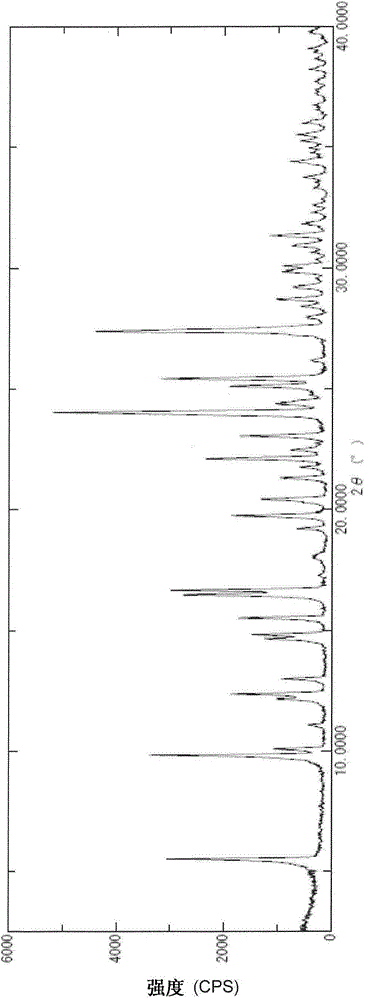
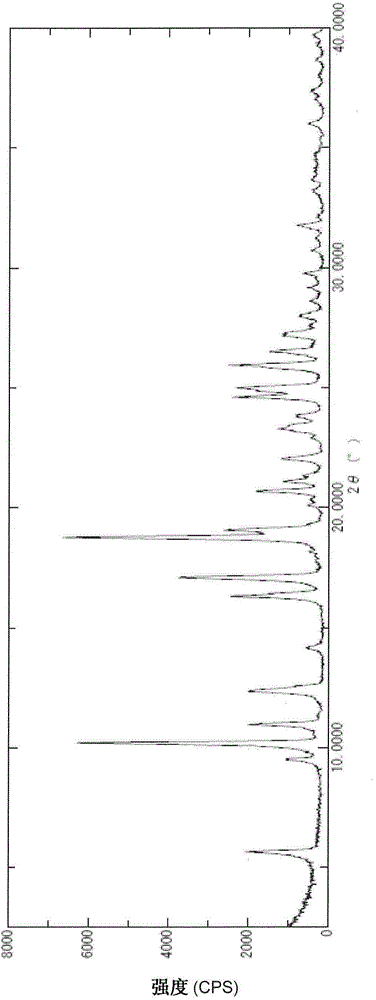
Image
Examples
Embodiment
[0276] Hereinafter, the method for producing the compound of formula (I) will be described in more detail based on examples. It should be noted that the present invention is not limited to the compounds described in the following Examples. In addition, the manufacturing method of the raw material compound is shown in the manufacturing example. In addition to the production methods described in the examples shown below, a combination of these production methods or methods obvious to those skilled in the art can be used to produce the compound of formula (I).
[0277] In addition, the following abbreviations may be used in Examples, Production Examples, and the following tables.
[0278] PEx: Manufacturing Example Number, Ex: Example Number, No.: Compound Number, Str: Structural Formula, DATA: Physical and Chemical Data (ESI+: ESI-MS[M+H] + Or ESI-MS[M] + ; ESI-: ESI-MS[M-H] - ; CI+: CI-MS[M+H] + ; EI: EI[M] + ; APCI / ESI+: APCI / ESI-MS[M+H] + Or APCI / ESI-MS[M] + (APCI / ESI refers to t...
manufacture example 1
[0291] Combine 8-hydroxy-2-methylimidazo[1,2-a]pyridine-3-carboxylic acid ethyl ester 500mg, 2,3-difluorobenzyl bromide 0.35ml, potassium carbonate 650mg N,N-dimethylformaldehyde A suspension of 8.6 ml of amide (DMF) was stirred at 60°C for 1 hour. The reaction mixture was left to cool to room temperature, and then water was added and the resulting solid was filtered out, and washed with water. The solid was washed with diisopropyl ether to obtain 650 mg of ethyl 8-[(2,3-difluorobenzyl)oxy]-2-methylimidazo[1,2-a]pyridine-3-carboxylate.
manufacture example 2
[0293] Add 4.3 ml of ethyl 2-chloro-3-oxobutyrate and 4.3 ml of triethylamine to a solution of 5.8 g of 3-(cyclohexylmethoxy)pyridine-2-amine in 100 ml of toluene, and stir overnight under heating and reflux . 1 ml of ethyl 2-chloro-3-oxobutyrate and 1 ml of triethylamine were added to the reaction mixture, and the mixture was stirred under reflux with heating for 6.5 hours. Water was added to the reaction mixture, and extraction was performed with ethyl acetate. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel column chromatography. Ethyl acetate and hexane were added to the obtained purified product, heated and stirred, and then stirred under ice cooling. The produced solid was collected by filtration to obtain 4.0 g of ethyl 8-(cyclohexylmethoxy)-2-methylimidazo[1,2-a]pyridine-3-carboxylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com