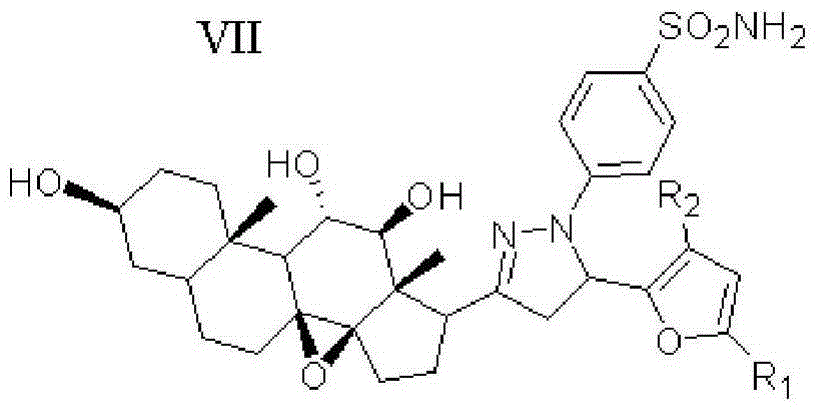
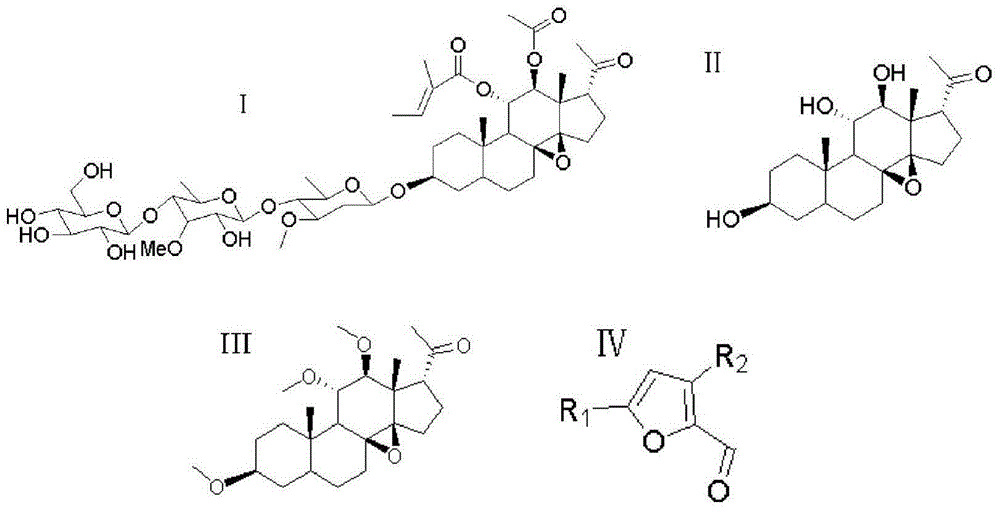
Furan skeleton-containing dihydropyrazolsulfanilamide C21 steroid sapogenin derivative, and preparation method and application thereof
A technology of dihydropyrazole sulfonamide and saponin aglycone, applied in the direction of medical preparations containing active ingredients, steroids, drug combinations, etc., can solve the problems of high toxicity, low biological activity and selectivity, and achieve low toxicity , good biological activity, novel structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: 4-(5-(2-furan)-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy)-(10a,12a- Dimethyl)-(1,2-cyclopentyl)-1,10a-b-epoxytetradecyl)-(4,5-dihydropyrazole))benzenesulfonamide (Compound 10) preparation of
[0048]
[0049] Under stirring at -20°C, add the corresponding intermediate 7 (10.0mmol) and dichloromethane (25mL) obtained in step 4 to a 50mL round bottom flask in turn, and gradually add boron tribromide (5mmol) dropwise to continue the reaction with stirring After 1 h, the reaction flask was transferred to room temperature, and the reaction was continued for 12 h. The reaction was tracked by TLC (developer VAcOEt:V n-hexane=1:2). After the reaction, it was filtered, the solid was washed with distilled water, and finally dried in vacuum. The obtained solid was dissolved in absolute ethanol for recrystallization and purification to obtain the crystalline target compound.
[0050] White crystals were obtained with a yield of 48.9%. m.p.196~197℃; 1H N...
Embodiment 2
[0051] Example 2: 4-(5-(2-(5-methyl-furan))-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy )-(10a,12a-dimethyl)-(1,2-cyclopentyl)-1,10a-b-epoxytetradecyl)-(4,5-dihydropyrazole)) Preparation of Benzenesulfonamide (Compound 11)
[0052]
[0053] The preparation method is the same as in Example 1 (using furan-2-carbaldehyde with different substituents from Example 1). White crystals were obtained with a yield of 44.2%. m.p.200~201℃; 1H NMR(DMSO-d6,300MHz)δ:8.03(d,J=7.5Hz,2H,ArH),7.73(d,J=7.6Hz,2H,ArH),6.88(s,2H ,NH2),6.17(d,J=5.2Hz,1H,CH),6.02(d,J=5.6Hz,1H,CH),5.58(t,J=4.4Hz,1H,CH),5.39(s, 2H, OH), 4.48(s, 1H, OH), 3.54(dd, J1=4.7, J2=4.2Hz, 1H, CH), 3.44(t, J=8.1Hz, 1H, CH), 3.34(d, J=6.8Hz, 1H, CH), 2.79(dd, J1=4.8, J2=5.1Hz, 2H, CH2), 2.20(s, 3H, CH3), 2.01(t, J=7.1Hz, 1H, CH) ,1.72~1.31(m,15H,CH and CH2),1.13(dd,J1=7.2Hz,J2=7.1Hz,1H,CH),0.84(s,3H,CH3),0.80(s,3H,CH3) .ESI-MS:626.8[M+H]+.Anal.Calcd for C33H43N3O7S:C,H,N.
Embodiment 3
[0054] Example 3: 4-(5-(2-(5-fluoro-furan))-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy) -(10a,12a-dimethyl)-(1,2-cyclopentyl)-1,10a-b-epoxy tetradecyl)-(4,5-dihydropyrazole))benzene Preparation of sulfonamide (compound 12)
[0055]
[0056] The preparation method is the same as in Example 1 (using furan-2-carbaldehyde with different substituents from Example 1). White crystals were obtained with a yield of 53.1%. m.p.215~216℃; 1H NMR (DMSO-d6, 300MHz) δ: 7.94(d, J=7.1Hz, 2H, ArH), 7.77(d, J=7.9Hz, 2H, ArH), 7.68(d, J =5.1Hz,1H,CH),6.87(s,2H,NH2),6.50(t,J=5.5Hz,1H,CH),5.58(t,J=5.3Hz,1H,CH),5.34(s, 2H, OH), 4.44(s, 1H, OH), 3.58(dd, J1=4.5, J2=4.1Hz, 1H, CH), 3.44(t, J=8.1Hz, 1H, CH), 3.34(d, J=6.8Hz, 1H, CH), 2.79(dd, J1=3.7, J2=3.4Hz, 2H, CH2), 2.01(t, J=7.1Hz, 1H, CH), 1.79~1.21(m, 15H, CH2 and CH), 1.14(dd, J1=7.2Hz, J2=7.1Hz, 1H, CH), 0.89(s, 3H, CH3), 0.84(s, 3H, CH3). ESI-MS: 630.7[M+ H]+.Anal.Calcd for C32H40FN3O7S:C,H,N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com