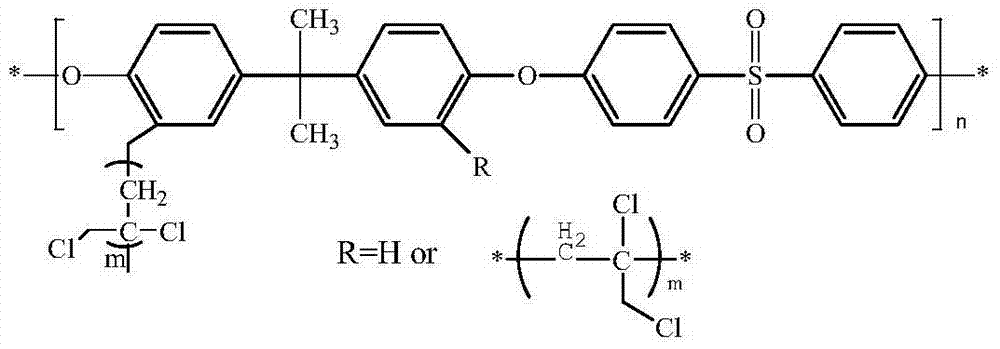
Alkaline anion exchange membrane and production method thereof
A technology of alkaline anion and exchange membrane, which is applied in the direction of electrical components, circuits, battery components, etc., and can solve the problems of increasing the internal stress of the membrane, hindering the rotation of molecular chains, and making the dry film brittle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
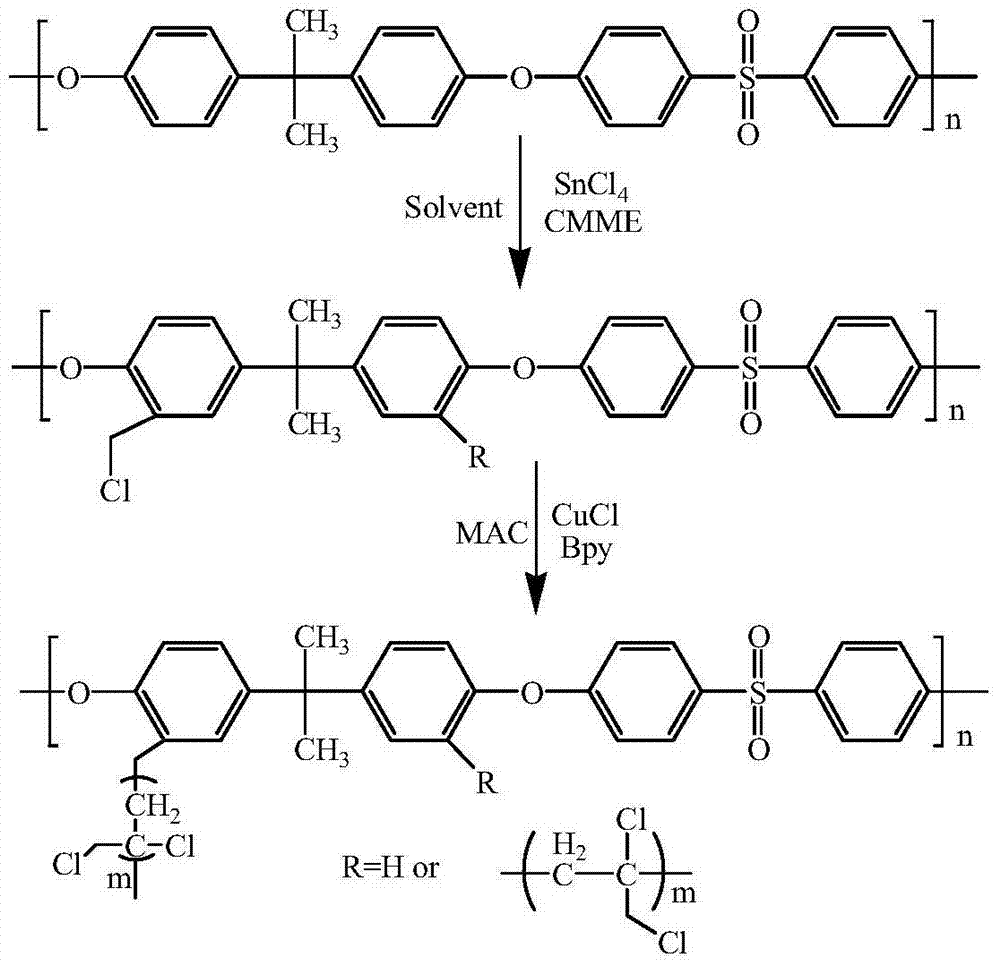
[0034] The chloromethylation reaction of polysulfone: the bisphenol A polysulfone of 1 weight unit is dissolved in the chloroform under nitrogen atmosphere, adds the tin tetrachloride (SnCl 4 ) and 1.7 weight units of chloromethyl methyl ether (CMME) were reacted at 55° C. for 12 hours. The product was poured into ethanol, filtered with suction, washed repeatedly with ethanol and deionized water, and then dried under vacuum at 50°C for 12 hours. Add 1 weight unit of chloromethylated bisphenol A polysulfone, 0.05 weight unit of cuprous chloride (CuCl), and 0.15 weight unit of 4,4'-bipyridine (Bpy) into the reactor, vacuumize, Charge nitrogen, switch repeatedly 5 times to ensure that the reactor is an inert gas atmosphere, add 25 weight units of N-methylpyrrolidone (NMP) to the reactor with a syringe under a nitrogen atmosphere, after dissolving, add 1 weight unit of 3-chloro - 2-Methyl-1-propene (MAC), after mixing well, react at 80°C for 24 hours, 36 hours, 48 hours; pour t...
Embodiment example 2
[0037] The chloromethylation reaction of polysulfone: the bisphenol A polysulfone of 1 weight unit is dissolved in the chloroform under nitrogen atmosphere, adds the tin tetrachloride (SnCl 4) and 1.7 weight units of chloromethyl methyl ether (CMME) were reacted at 55° C. for 12 hours. The product was poured into ethanol, filtered with suction, washed repeatedly with ethanol and deionized water, and then dried under vacuum at 50°C for 12 hours. Add 1 weight unit of chloromethylated bisphenol A polysulfone, 0.03 weight unit of cuprous chloride (CuCl), and 0.09 weight unit of 4,4'-bipyridine into the reactor, vacuumize and fill with nitrogen, Switch repeatedly 5 times to ensure that the reactor is an inert gas atmosphere, add 25 weight units of N-methylpyrrolidone (NMP) into the reactor with a syringe under a nitrogen atmosphere, and after dissolving, add 1 weight unit of 3-chloro-2- Methyl-1-propene, after fully mixed, reacted at 80°C for 24 hours, 36 hours, 48 hours; pour t...
Embodiment example 3
[0040] The chloromethylation reaction of polysulfone: the bisphenol A polysulfone of 1 weight unit is dissolved in the chloroform under nitrogen atmosphere, adds the tin tetrachloride (SnCl 4 ) and 1.7 weight units of chloromethyl methyl ether (CMME) were reacted at 55° C. for 12 hours. The product was poured into ethanol, filtered with suction, washed repeatedly with ethanol and deionized water, and then dried under vacuum at 50°C for 12 hours. Add 1 weight unit of chloromethylated bisphenol A polysulfone, 0.02 weight unit of cuprous chloride (CuCl), and 0.06 weight unit of 4,4'-bipyridine (Bpy) into the reactor, vacuumize, Charge nitrogen, switch repeatedly 5 times to ensure that the reactor is an inert gas atmosphere, add 25 weight units of N-methylpyrrolidone (NMP) to the reactor with a syringe under a nitrogen atmosphere, after dissolving, add 1 weight unit of 3-chloro - 2-Methyl-1-propene (MAC), after mixing well, react at 80°C for 24 hours, 36 hours, 48 hours; pour t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com