A kind of fluorescent rt-PCR primer, probe and test kit and detection method for detecting mers
An RT-PCR and fluorescent probe technology, applied in the field of early clinical diagnosis of MERS-CoV, can solve the problems of increased epidemic risk and limited infectivity of MERS, so as to reduce the fatality rate, shorten the detection time, and reduce the sensitivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
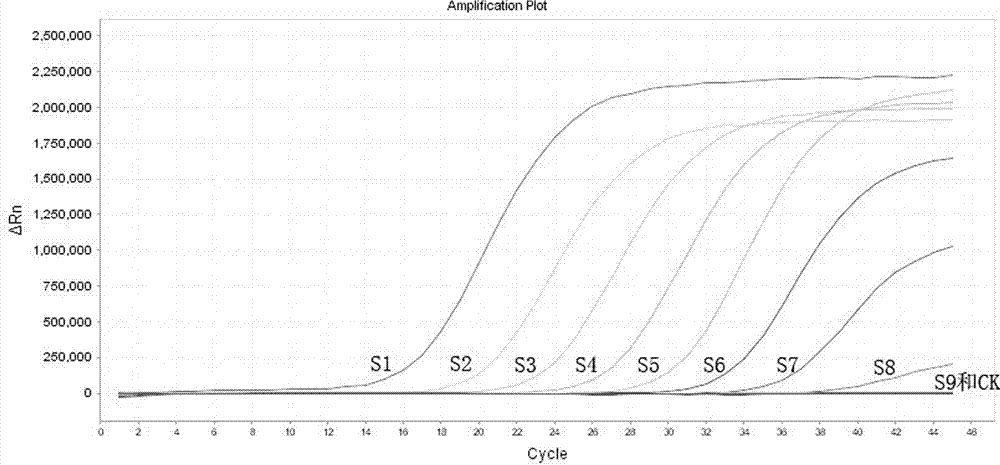
[0063] The linear experiment of the first pair of probes of embodiment 1 kit
[0064] The first pair of target probes is labeled with FAM fluorescent groups. The linear reference product of the FAM channel of the kit consists of plasmids containing the first pair of target fragments. The positive plasmids are serially diluted 10 times with TE buffer. The following gradient solution was obtained (S1: 1.0 x 10 10 copies / ml, S2: 1.0×10 9 copies / ml, S3: 1.0×10 8 copies / ml, S4: 1.0×10 7 copies / ml, S5: 1.0×10 6 copies / ml, S6: 1.0×10 5 copies / ml, S7: 1.0×10 4 copies / ml, S8: 1.0×10 3 copies / ml, S9: 1.0×10 2 copies / ml,) as a linear reference for the FAM channel, the experiment shows that the kit is at 1.0×10 10 copies / ml to 1.0×10 3 There is a good linear relationship between copies / ml, the experimental results refer to figure 1 .
Embodiment 2
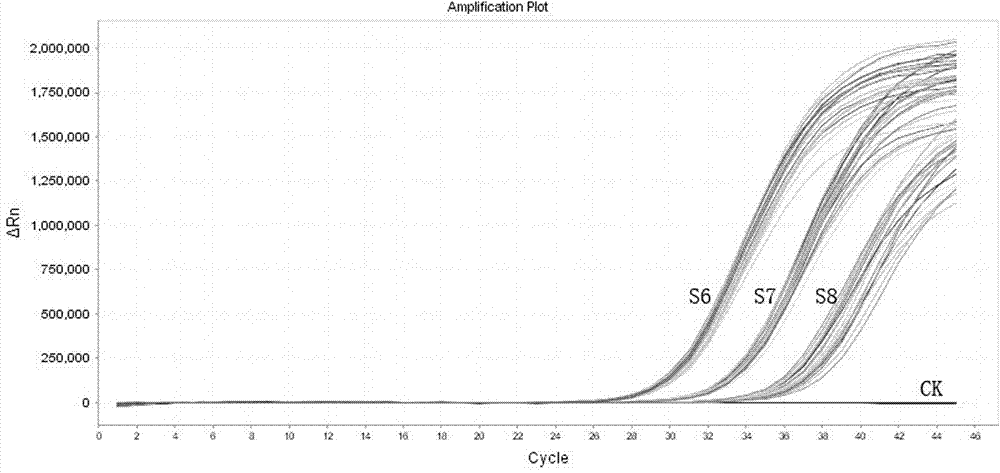
[0065] The minimum detection limit experiment of embodiment 2 kit
[0066] This experiment validates the lowest detection limit of the kit, using S6 in the linear reference product of the FAM channel: 1.0×10 5 copies / ml, S7: 1.0×10 4 copies / ml, S8: 1.0×10 3 Positive plasmids with equal concentrations of copies / ml are used as the detection limit reference product of the first pair of probes. This experiment shows that the detection limit of the kit reaches 5 copies / reaction. The experimental results refer to figure 2 .
Embodiment 3
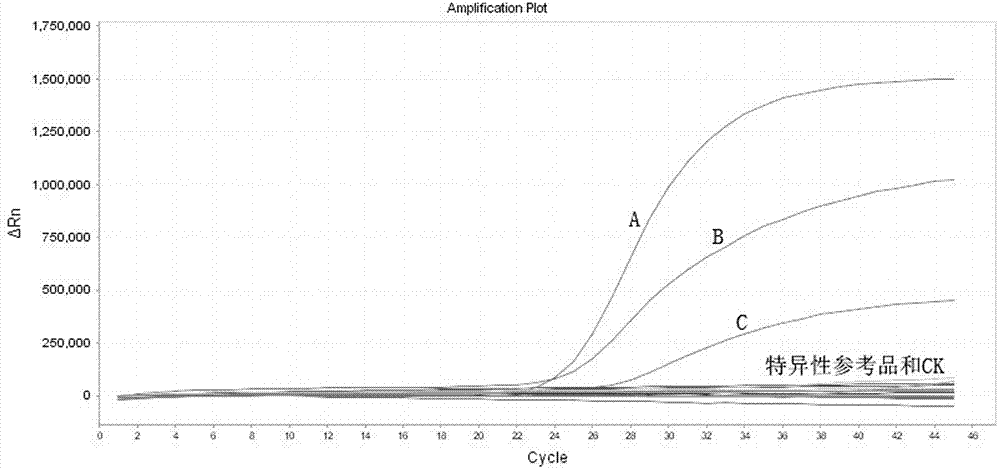
[0067] Embodiment 3 The specificity experiment of kit
[0068] In this experiment, common pathogens with the same infection site as MERS-CoV were selected as specific reference substances, and inactivated clinical positive samples were used. Specific reference products are influenza A H1N1 virus, H7N9 avian influenza virus, influenza A H3N1 virus, influenza A H3N2 virus, norovirus type GI, norovirus type GII, parainfluenza virus type 1, parainfluenza virus Type 2, coronavirus 229E, enteric adenovirus, influenza virus type A. Use the universal virus RNA extraction kit developed by Shenzhen Yirui Biotechnology Co., Ltd. to extract the RNA in the above inactivated samples as a template for experiments. The experimental data shows that none of the 8 specific reference products has a typical "S" type The amplification curve shows that the specificity of the kit is good, and the experimental results refer to image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com