Application of strigolactone analog in preparation of anti inflammatory medicine
A technology of strigolactone and anti-inflammatory drugs, which is applied in the direction of anti-inflammatory agents, drug combinations, antipyretics, etc., and can solve the problems of unreported anti-inflammatory effects of strigolactones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0134] The preparation method of the raw material used in the embodiment is as follows:
[0135] 3,3a,4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one (4):
[0136] Take 1-indanone (5.0g, 37.88mmol) and dimethyl carbonate (13.6g, 151.11mmol) and dissolve in 15ml of anhydrous tetrahydrofuran, slowly add dropwise to 20ml of anhydrous tetrahydrofuran, sodium hydride (2.0g, 83.33mmol) In the suspension solution, protected by nitrogen, reflux at 65° C. and stir for 1.5 hours to react. Then 10 ml of tetrahydrofuran solution of ethyl bromoacetate (9.5 g, 56.89 mmol) was added, and the reaction was continued for 2 hours. After TLC monitors that the reaction of raw materials is complete, add saturated ammonium chloride solution to quench the reaction, remove the solvent under reduced pressure, extract with ethyl acetate, dry the organic phase, filter, concentrate, and purify by silica gel column chromatography to obtain 2-ethoxycarbonylmethyl- Methyl 1-oxo-indene-2-carboxylate (1) was a w...
Embodiment 1
[0142] Example 1 Preparation of Compounds 1A-1F
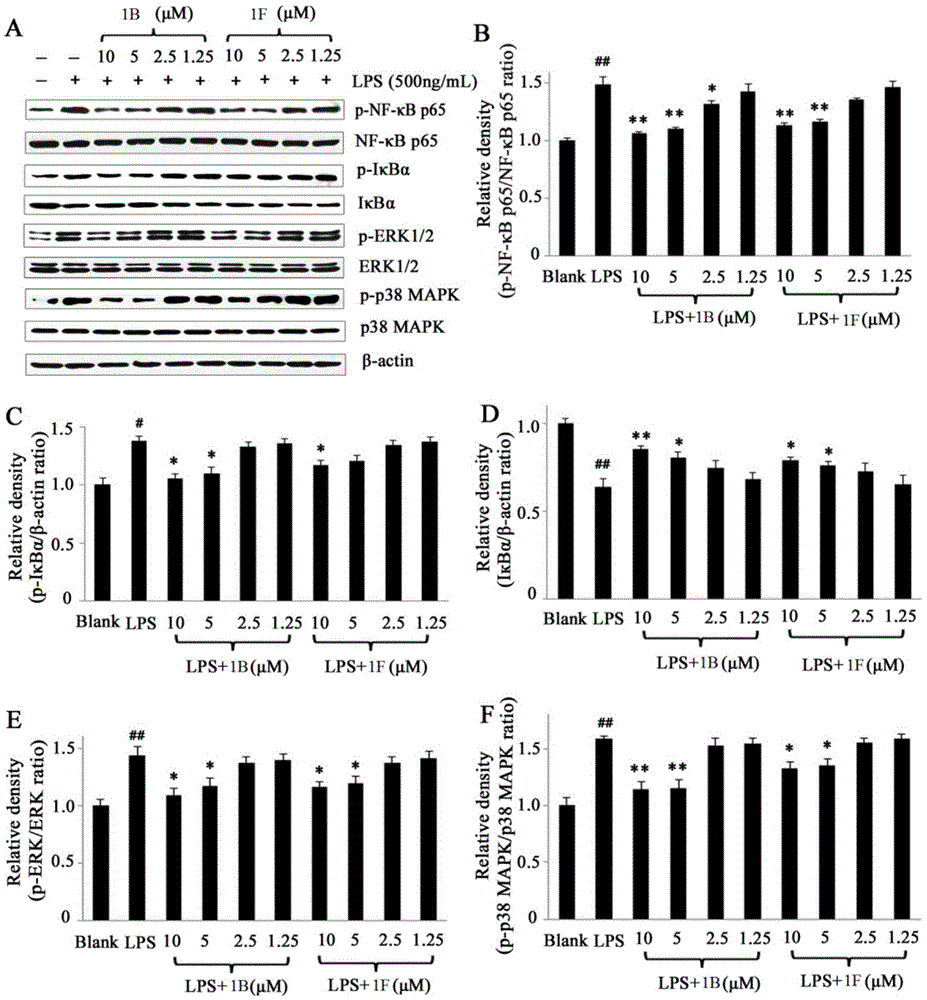
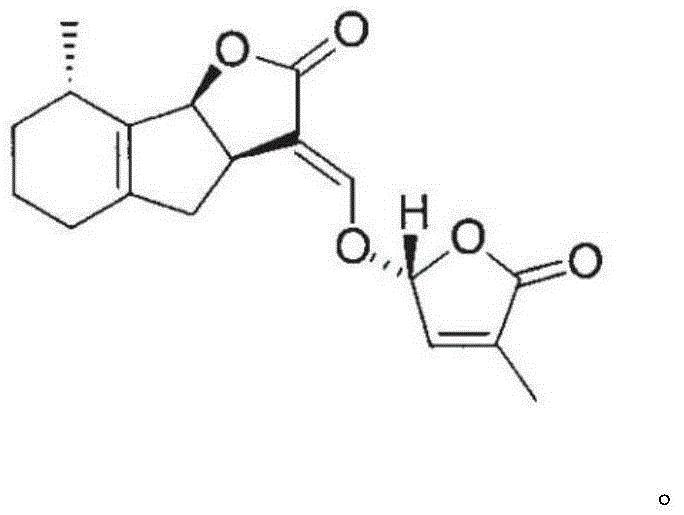
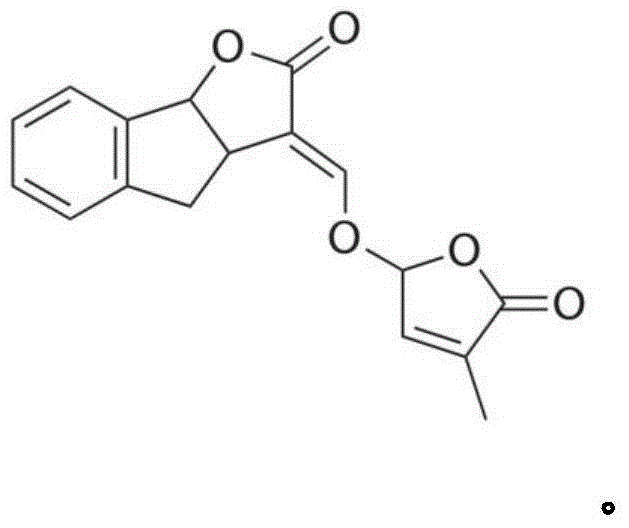
[0143] Dissolve 3,3a, 4,8b-tetrahydro-2H-indeno[1,2-b]furan-2-one (1g, 5.75mmol) and methyl formate (1.96g, 32.7mmol) in 8ml of anhydrous Add this solution to a solution of sodium metal (0.26g, 11.36mmol) in tetrahydrofuran (5ml), and stir at room temperature for 2 hours under nitrogen protection. After the complete reaction of the raw materials was monitored by TLC, 5 ml of a tetrahydrofuran solution of 5-bromo-3-methyl-5H-furan-2-one (0.86 g, 4.9 mmol) was added, and stirring was continued for 2 hours. After the reaction was completed, ethanol was added to quench the reaction, the solvent was removed under reduced pressure, extracted with ethyl acetate, the organic phase was dried, filtered, concentrated, and separated by silica gel column chromatography to obtain diastereoisomers 1A and 1D. 1A, 1D were resolved by chiral column to obtain 1B, 1C and 1E, 1F respectively.
[0144]
Embodiment 2
[0145] Example 2 Preparation of Compounds 1a-1f
[0146] Dissolve 1A or (1B-1F) in ethyl acetate (10ml), add Pd / C (10%) hydrogen and react at room temperature for 3 hours. The reaction solution was filtered, and the filtrate was concentrated and separated by silica gel column chromatography to obtain 1a or (1b~1f)
[0147]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com