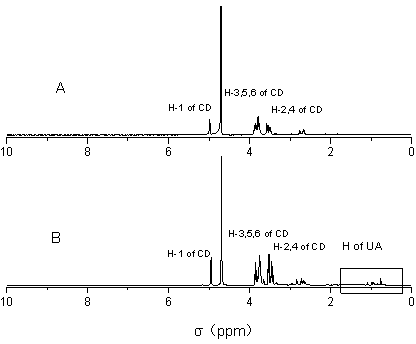
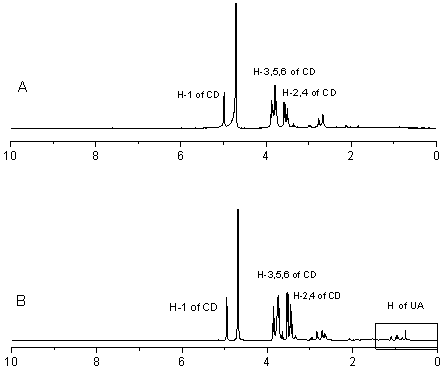
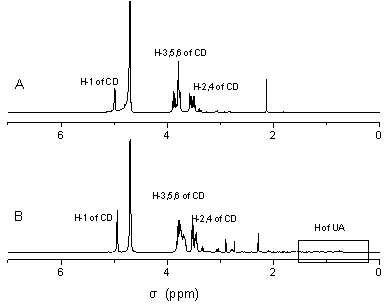
Ursolic acid and amine cyclodextrin clathrate compound
A technology of cyclodextrin and ursolic acid, which is applied in the field of pharmaceuticals, can solve the problems of low bioavailability and poor water solubility, and achieve the effects of overcoming poor water solubility, good stability and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of inclusion compound of ursolic acid and mono-(6-ethylenediamino-6-deoxy)-β-cyclodextrin
[0027] (1) Preparation of single 6-OTs-β-CD
[0028] Take 210 g of β-cyclodextrin recrystallized with water, add it in batches to a three-necked bottle filled with 1300 mL of water, stir at room temperature, weigh 17.2 g of sodium hydroxide and dissolve it in 50 mL of water, and then slowly oxidize the hydroxide Sodium aqueous solution was added dropwise to the suspension of β-cyclodextrin until the reaction solution gradually became clear, and continued to stir for 1.5 h. After the cyclodextrin in the reaction solution was completely dissolved and became clear, acetonitrile containing 26.0 g of p-toluenesulfonyl chloride (80 mL) solution was slowly added dropwise to the reaction solution (dropped in about 30 minutes), continued to stir for 2 h, filtered off the insoluble matter, and the filtrate was adjusted to pH = 7.5 with 2 mol / L hydrochloric acid, at t...
Embodiment 2
[0034] Example 2: Preparation of inclusion compound of ursolic acid and mono-(6-diethylenetriamine-6-deoxy)-β-cyclodextrin
[0035] (1) The preparation method of single 6-OTs-β-CD is the same as step (1) of Example 1;
[0036] (2) Preparation of mono-(6-diethylenetriamine-6-deoxy)-β-cyclodextrin (DETA-β-CD)
[0037] Dissolve 3.0 g (2.3 mmol) of mono-6-OTs-β-CD in 20 mL of dry diethylenetriamine, stir at room temperature until completely dissolved, and react at 80°C for 10 h under nitrogen protection. After the reaction is completed, Slowly drop the reaction solution into 400 mL of acetone, stir at room temperature for 30 min, remove the filtrate by suction filtration, collect the white precipitate, dissolve the precipitate in a small amount of water, drop it into 300 mL of acetone again, stir for 0.5 h, and then suction filter The white precipitate was collected and dried in a vacuum oven. The resulting solid powder was ground and added to 200 mL of ethanol. After ultras...
Embodiment 3
[0041] Example 3: Preparation of inclusion compound of ursolic acid and mono-(6-amino-6-deoxy)-β-cyclodextrin
[0042] (1) The preparation method of single 6-OTs-β-CD is the same as step (1) of Example 1;
[0043] (2) Single-6-N 3 -β - Preparation of CDs
[0044] Accurately weigh 10 g (8 mmol) of mono-6-OTs-β - CD was dissolved in 35 mL DMF, followed by the addition of 1.5 g NaN 3 Stir well, heat the reaction at 75°C for 12 h, and check whether the raw material is completely reacted by TLC. After the reaction was complete, the reaction solution was slowly added dropwise to 400 mL of acetone, stirred at room temperature, and suction filtered to obtain a white solid. The obtained white crude product was dried for 24 hours and then uniformly placed in 400 mL of acetone, followed by ultrasonication for 10 min and suction filtration to obtain Pure mono-6-N 3 -β - CD, 90% yield. The product is characterized as follows: 1 H NMR (500 MHz, D 2 O, δ ppm): 5.77(s, 14H, 2, 3-OH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com