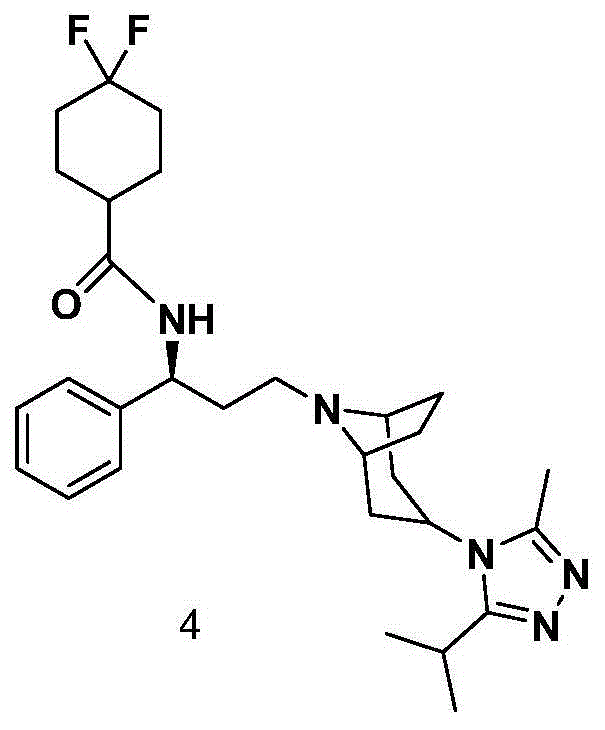
Preparation method of CCR5 antagonist
A technology of reducing agent and organic solvent, applied in the field of preparation of CCR5 antagonists, can solve problems to be improved, etc., and achieve the effects of ideal reaction efficiency, improved reaction speed and high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
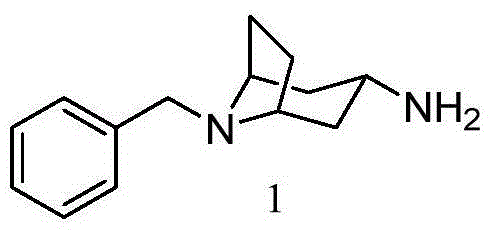
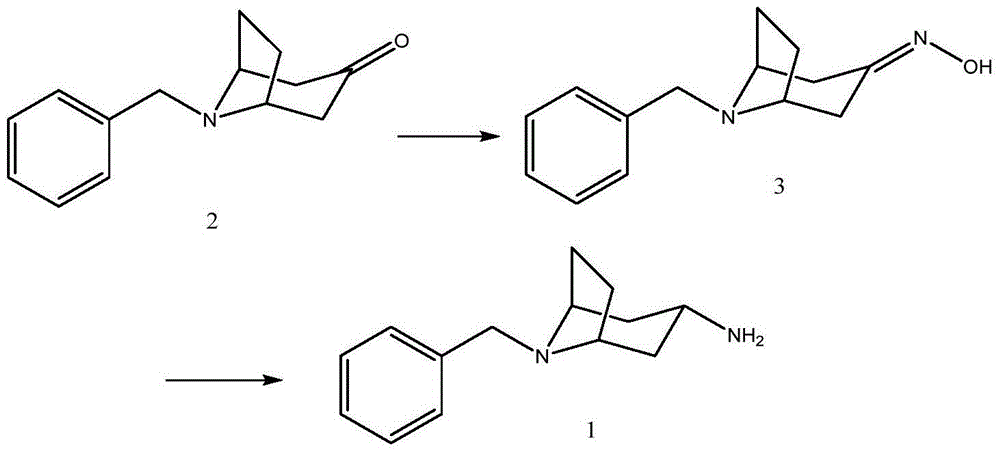
[0052] Embodiment 1: the preparation of the compound shown in formula 1
[0053]
[0054] 215 mg compound 2, 770 mg NH 4 OAc, 44 mg NaBH 3 CN and 3 milliliters of methanol were mixed, and the resulting reaction system was stirred and reacted at room temperature for 48 hours. After the disappearance of the starting material was detected by TLC, 120 microliters of concentrated hydrochloric acid was added to the reaction system to quench the reaction, and then the reaction mixture was concentrated under reduced pressure. Obtain a white solid, then dissolve the white solid in 25 ml of water, and adjust the pH value of the solution to about 10 with solid KOH, extract the aqueous phase with chloroform (16 ml, 8 ml) twice, combine the chloroform solution, add anhydrous sodium sulfate After drying and concentration, 194 mg of the compound represented by Formula 1 was obtained, with a yield of 89.8%, and a purity of 98.8% as determined by HPLC.
[0055] LCMS(ESI)m / z:216(M+1)
Embodiment 2
[0056] Embodiment 2: the preparation of compound shown in formula 1
[0057] 215 mg compound 2, 960 mg (NH 4 ) 2 CO 3 , 31.4 mg NaBH 3 CN and 3 milliliters of methanol were mixed, and then the resulting reaction system was stirred and reacted at room temperature for 45 hours. After the disappearance of the starting material was detected by TLC, 110 microliters of concentrated hydrochloric acid was added to the reaction system to quench the reaction, and then the reaction mixture was concentrated under reduced pressure. Obtain a white solid, then dissolve the white solid in 25 ml of water, and adjust the pH value of the solution to about 10 with solid KOH, extract the aqueous phase with chloroform (16 ml, 8 ml) twice, combine the chloroform solution, add anhydrous sodium sulfate After drying and concentration, 199 mg of the compound represented by Formula 1 was obtained, with a yield of 92.1%, and a purity of 97.0% as determined by HPLC.
Embodiment 3
[0058] Embodiment 3: the preparation of compound shown in formula 1
[0059] 215 mg compound 2, 770 mg NH 4 OAc, 31.4 mg NaBH 3 CN and 2 milliliters of methanol were mixed, and the resulting reaction system was stirred and reacted at room temperature for 48 hours. After the disappearance of the starting material was detected by TLC, 120 microliters of concentrated hydrochloric acid was added to the reaction system to quench the reaction, and then the reaction mixture was concentrated under reduced pressure. Obtain a white solid, then dissolve the white solid in 25 ml of water, and adjust the pH value of the solution to about 10 with solid KOH, extract the aqueous phase with chloroform (16 ml, 8 ml) twice, combine the chloroform solution, add anhydrous sodium sulfate After drying and concentration, 199 mg of the compound represented by Formula 1 was obtained, with a yield of 92.1%, and a purity of 98.5% as determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com