Method for preparing flavonoids compounds in camellia seed shells by high-speed counter-current chromatography
A technology of high-speed countercurrent chromatography and flavonoids, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Preparation of flavonoids in camellia oleifera seed shell
[0018] Camellia oleifera seed shells in this example were purchased from Hongyuan Agriculture and Forestry Development Co., Ltd., Huaihua City, Hunan Province.
[0019] 1. Preparation of raw material (camellia oleifera seed husk extract) for high-speed countercurrent (HSCCC) loading
[0020] Take 200 g of Camellia oleifera seed shell powder that has been crushed through a 10-mesh sieve, extract three times at 60°C with 70% ethanol of 6 times the weight of Camellia oleifera seed shell powder, the first time is 60 minutes, and the last two times are 30 minutes each, and the extract is collected , combined, filtered, concentrated under reduced pressure at 55°C to recover ethanol, and the obtained water layer (extract) was adsorbed by a chromatographic column equipped with 300ml D-101 macroporous adsorption resin at a flow rate of 3BV / h, and left to stand for 30min after adsorption , first rinse with 900...
Embodiment 2
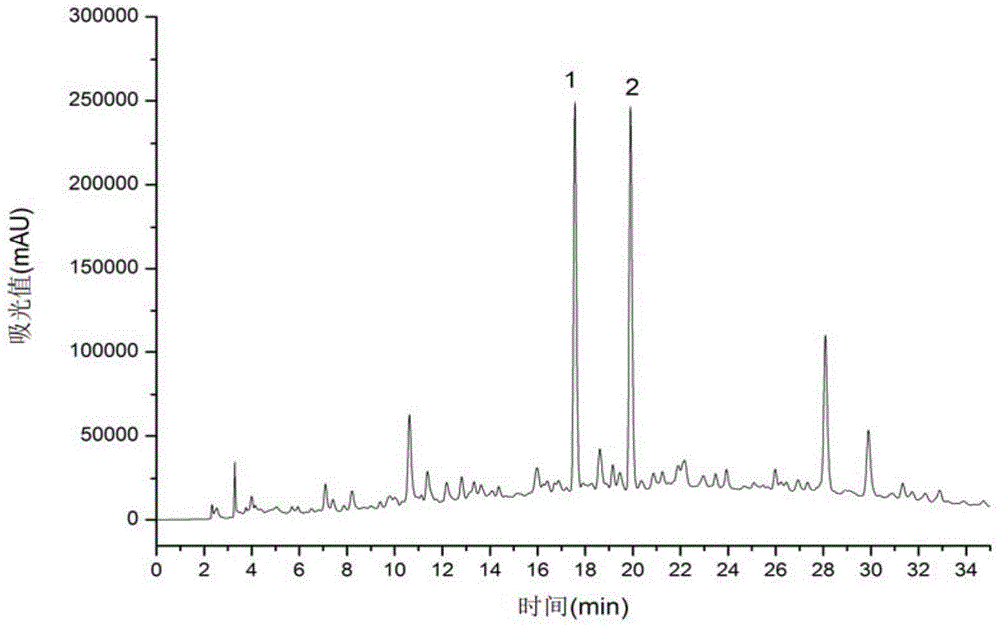
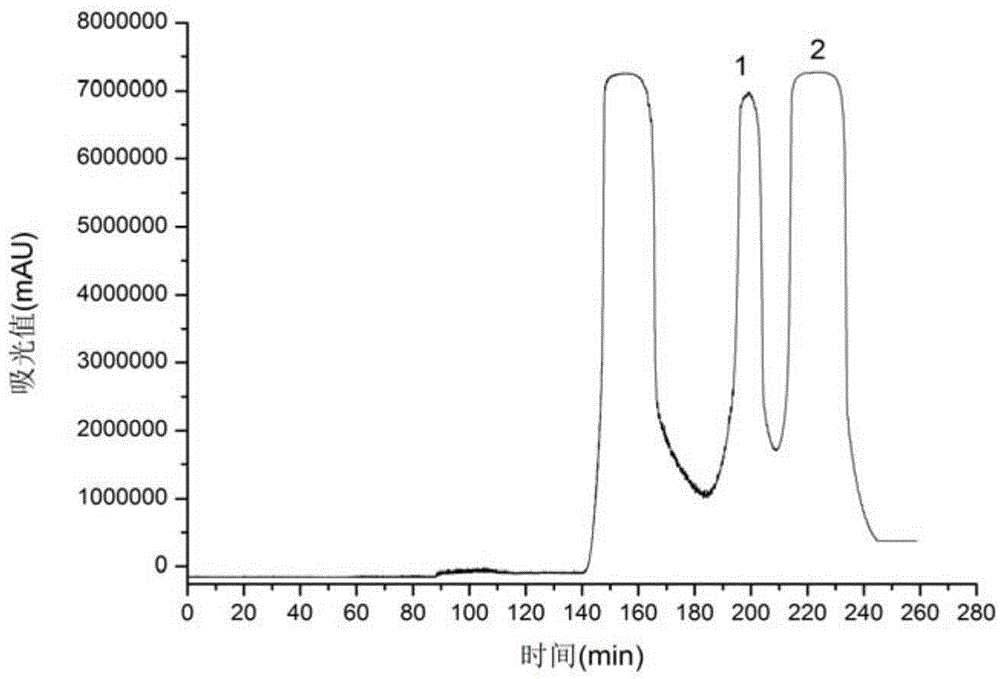
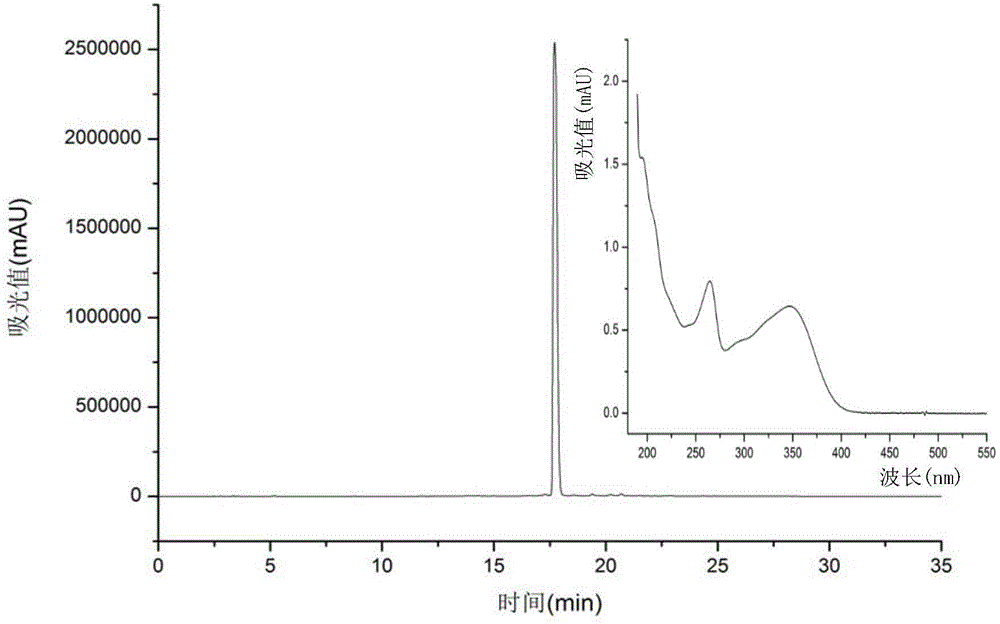
[0033] see in conjunction image 3 and Figure 4 According to the chromatogram of HPLC (analytical conditions as above) at 254nm, the purity of the compound was calculated by the peak area normalization method, the purity of compound 1 was 99%, and the purity of compound 2 was 93%. The structural identification of embodiment 2 compound
[0034] The structures of the above two compounds were identified by mass spectrometry and nuclear magnetic resonance.
[0035] Compound 1: light yellow powder, molecular weight 756.21, component formula C 33 h 40 o 20 ,according to 1 H and 13 C NMR data, combined with the literature, identified the compound as: kaempferol-3-O-[β-D-glucopyranosyl-(1→3)-α-L-rhamnopyranose-(1→6 )-β-D-glucopyranoside], its structure is as follows, and the NMR signal assignment is shown in Table 2.
[0036]
[0037] Table 2 The H NMR and C NMR spectra of compound 1
[0038]
[0039]
[0040] Compound 2: light yellow powder, molecular weight 730.20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com