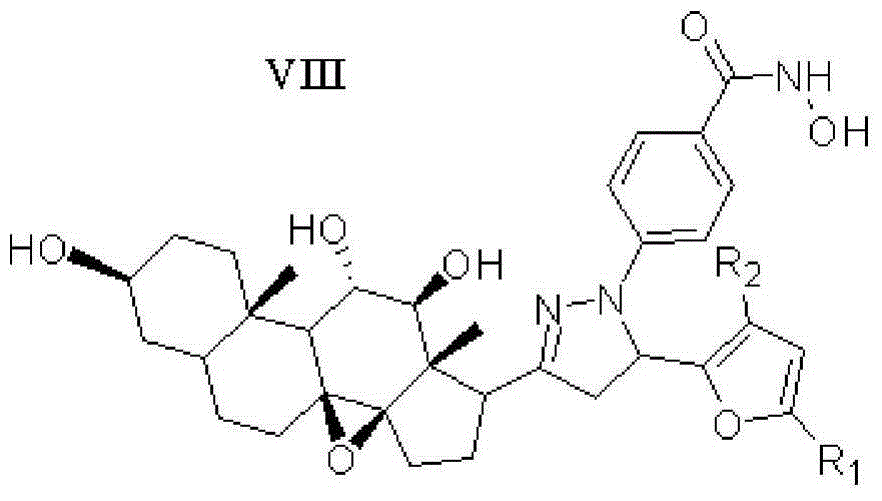
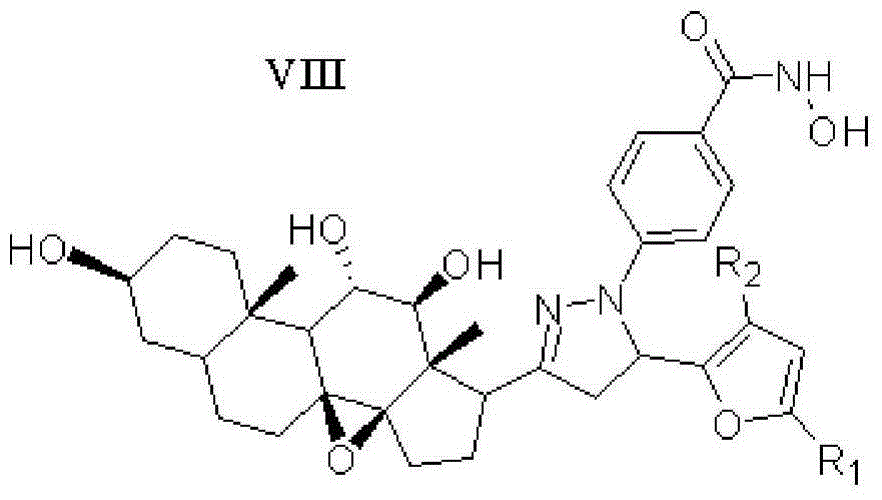
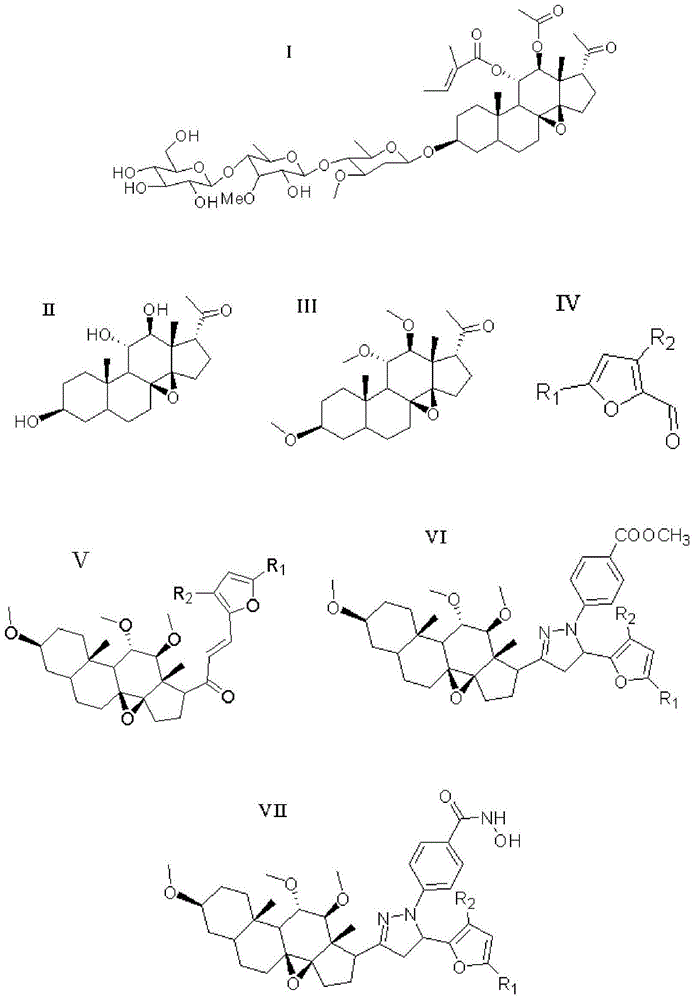
Furan skeleton included 2H-pyrazole hydroxamic acid C21 steroid saponin aglycone derivative and preparation method and application thereof
A technology of dihydropyrazole hydroxamic acid and saponin aglycone, which is applied in the field of dihydropyrazole hydroxamic acid C21 steroidal saponin aglycon derivatives, can solve the problems of low biological activity and selectivity, and high toxicity, and achieve good Biological activity, low toxicity, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: 4-(5-(2-furan)-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy)-(10a,12a- Dimethyl)-(1,2-cyclopentyl)-1,10a-b-epoxytetradecyl)-(4,5-dihydropyrazole))benzoylhydroxamic acid ( Compound 15) Preparation
[0053]
[0054] Under stirring at -20°C, add the corresponding intermediate 12 (10.0mmol) and dichloromethane (25mL) obtained in step 5 to a 50mL round bottom flask in turn, and gradually add boron tribromide (5mmol) dropwise to continue the reaction with stirring After 1 h, the reaction flask was transferred to room temperature, and the reaction was continued for 12 h. The reaction was tracked by TLC (developer VAcOEt:V n-hexane=1:2). After the reaction, it was filtered, the solid was washed with distilled water, and finally dried in vacuum. The obtained solid was dissolved in absolute ethanol for recrystallization and purification to obtain the crystalline target compound.
[0055] White crystals were obtained with a yield of 48.9%. m.p.195~197℃; 1...
Embodiment 2
[0056] Example 2: 4-(5-(2-(5-methyl-furan))-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy )-(10a,12a-dimethyl)-(1,2-cyclopentyl)-1,10a-b-epoxytetradecyl)-(4,5-dihydropyrazole)) Preparation of Benzoylhydroxamic Acid (Compound 16)
[0057]
[0058] The preparation method is the same as in Example 1 (using furan-2-carbaldehyde with different substituents from Example 1). White crystals were obtained with a yield of 44.2%. m.p.200~201℃; 1H NMR(DMSO-d6,300MHz)δ:10.54(s,1H,OH),8.03(d,J=7.5Hz,2H,ArH),7.73(d,J=7.6Hz,2H ,ArH),7.71(s,1H,NH),6.17(d,J=5.2Hz,1H,CH),6.02(d,J=5.6Hz,1H,CH),5.58(t,J=4.4Hz, 1H, CH), 5.39(s, 2H, OH), 4.48(s, 1H, OH), 3.54(dd, J1=4.7, J2=4.2Hz, 1H, CH), 3.44(t, J=8.1Hz, 1H, CH), 3.34(d, J=6.8Hz, 1H, CH), 2.79(dd, J1=4.8, J2=5.1Hz, 2H, CH2), 2.20(s, 3H, CH3), 2.01(t, J=7.1Hz, 1H, CH), 1.72~1.31(m, 15H, CH and CH2), 1.13(dd, J1=7.2Hz, J2=7.1Hz, 1H, CH), 0.84(s, 3H, CH3) ,0.80(s,3H,CH3).ESI-MS:606.7[M+H]+.Anal.Calcd for C34H43N3O7:C,H,N.
Embodiment 3
[0059] Example 3: 4-(5-(2-(5-fluoro-furan))-3-((3aR,4aS,8S,10aS,11S,12S,12aS)-(8,11,12-trihydroxy) -(10a,12a-dimethyl)-(1,2-cyclopentyl)-1,10a-b-epoxy tetradecyl)-(4,5-dihydropyrazole))benzene Preparation of Formylhydroxamic Acid (Compound 17)
[0060]
[0061]The preparation method is the same as in Example 1 (using furan-2-carbaldehyde with different substituents from Example 1). White crystals were obtained with a yield of 52.8%. m.p.213~214℃; 1H NMR(DMSO-d6,300MHz)δ:10.50(s,1H,OH),7.94(d,J=7.1Hz,2H,ArH),7.71(t,J=6.8Hz,3H , CH and NH), 7.68(d, J=7.9Hz, 2H, ArH), 6.50(t, J=5.5Hz, 1H, CH), 5.62(t, J=5.3Hz, 1H, CH), 5.34( s,2H,OH),4.44(s,1H,OH),3.58(dd,J1=4.5,J2=4.1Hz,1H,CH),3.44(t,J=8.1Hz,1H,CH),3.34( d, J=6.8Hz, 1H, CH), 2.79(dd, J1=3.7, J2=3.4Hz, 2H, CH2), 2.01(t, J=7.1Hz, 1H, CH), 1.79~1.21(m, 15H, CH2and CH), 1.14(dd, J1=7.2Hz, J2=7.1Hz, 1H, CH), 0.92(s, 3H, CH3), 0.85(s, 3H, CH3).ESI-MS: 610.7[M +H]+.Anal.Calcd for C33H40FN3O7:C,H,N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com