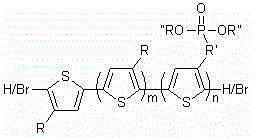
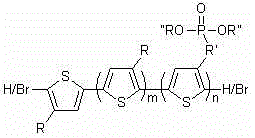
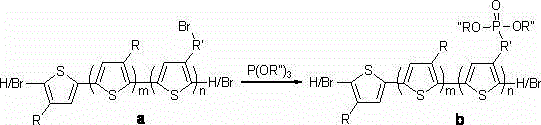Block copolymer of polyalkylthiophene and alkyl phosphite alkyl thiophene, and preparation method thereof
A technology of polyphosphite alkyl thiophene and block copolymer is applied in the field of polymer materials, which can solve the problems of low photoelectric conversion efficiency of polymer photovoltaic cells, achieve good alcohol solubility and processability, and improve efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: block copolymer 1 The synthesis of wherein R and R' are hexyl, R'' is ethyl. The number-average molecular weight of poly-3-hexylthiophene-block-poly-3-phosphite hexylthiophene is 13400, and the molecular weight distribution is 1.17. The number average molecular weight of hexylthiophene was 8000.
[0022] Step (1) 2-Bromo-5-iodo-3-alkylthiophene, the compound in the above reaction a One of the starting monomers, prepared according to existing conventional methods (see literature A. Yokoyama, R. Miyakoshi, T. Yokozawa. Macromolecules, 2004, 37(4): 1169-1171).
[0023] (2) 2-Bromo-5-iodo-3-(n-bromoalkyl)thiophene, the compound in the above reaction a Another starting monomer of , prepared according to existing conventional methods (see literature S. Miyanishi, K. Tajima, K. Hashimoto. Macromolecules, 2009, 42(5): 1610-1618).
[0024] (3) Reference patent method for the preparation of poly-3-hexylthiophene-block-poly-3-phosphite hexylthiophene (Chinese paten...
Embodiment 2
[0028] Example 2: block copolymer 2The synthesis of wherein R and R' are hexyl, R'' is ethyl. The number average molecular weight of poly-3-hexylthiophene-block-poly-3-phosphite hexylthiophene is 11400, and the molecular weight distribution is 1.16. The number average molecular weight of hexylthiophene is 4100.
[0029] Steps (1), (2) are the same as in Example 1.
[0030] (3) Reference patent method for the preparation of poly-3-hexylthiophene-poly-3-phosphite hexylthiophene (Chinese patent, patent number: ZL201210362669.6):
[0031] All the polymerization reactions were carried out under anhydrous and oxygen-free conditions, and the solvent tetrahydrofuran was refluxed with sodium to remove water. Under a nitrogen atmosphere, 1.6125 ml (7.2 mmol) of 2-bromo-5-iodo-3-hexylthiophene was dissolved in anhydrous tetrahydrofuran and cooled in an ice-water bath at 0 oC, then 3.6 ml (7.2 mmol) of iso Propylmagnesium chloride, stirred for 30 minutes and then warmed to 35 oC, tr...
Embodiment 3
[0034] Example 3: block copolymer 3 The synthesis of wherein R and R' are hexyl, R'' is ethyl. The number-average molecular weight of poly-3-hexylthiophene-block-poly-3-phosphite hexylthiophene is 16400, and the molecular weight distribution is 1.17. The number average molecular weight of hexylthiophene is 13100.
[0035] Steps (1), (2) are the same as in Example 1.
[0036] (3) Reference patent method for the preparation of poly-3-hexylthiophene-block-poly-3-phosphite hexylthiophene (Chinese patent, patent number: ZL201210362669.6):
[0037] All the polymerization reactions were carried out under anhydrous and oxygen-free conditions, and the solvent tetrahydrofuran was refluxed with sodium to remove water. Under a nitrogen atmosphere, 0.5375 ml (2.4 mmol) of 2-bromo-5-iodo-3-hexylthiophene was dissolved in anhydrous tetrahydrofuran and cooled in an ice-water bath at 0 oC, then 1.2 ml (2.4 mmol) of iso Propylmagnesium chloride, stirred for 30 minutes and then warmed to 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com