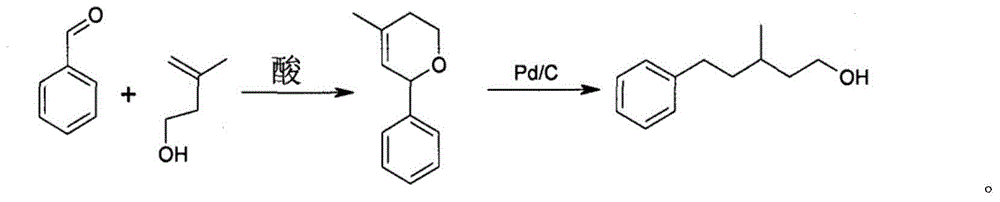
Process for preparing benorilate amyl alcohol
A preparation process, the technology of phenphenoperol, which is applied in hydrogenation preparation, organic chemistry, etc., can solve the problems of high cost, difficult cost reduction, and unsatisfactory selectivity, and achieve high yield, high hydrogenation yield, and promotion value high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
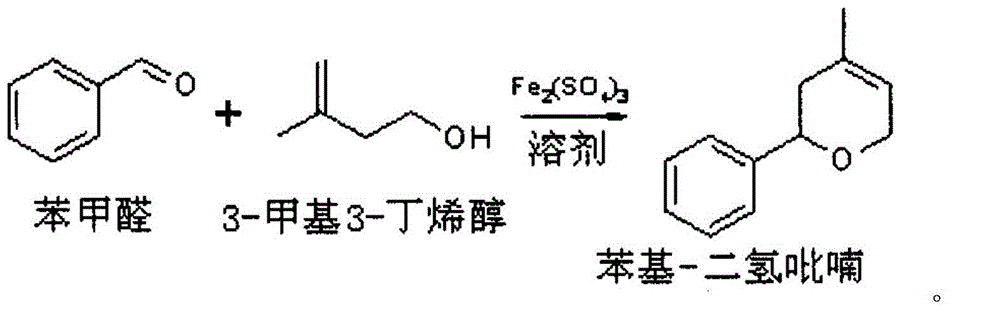
[0029] Step A: Take one 2000mL four-necked flask as a reaction kettle, add 400g of benzaldehyde, 400g of tert-butylbenzene, and 1g of iron sulfate. Turn on the stirring, raise the temperature of the materials in the kettle and control the reaction temperature to 150-155°C, add 350g of prenyl alcohol to the reaction kettle dropwise through the dropping funnel, as the addition proceeds, the reaction will produce water, and the produced water will be separated by a water separator. The water is removed, and the steamed upper oil phase is returned to the reactor.
[0030] Step B: After the dropwise addition, continue to reflux to separate the water. When the water is divided to 67mL, stop the water separation, drop to 50°C, and wash with water. The pH of the water washing must be greater than 6. After the pH is qualified, remove the water to obtain 1070g of the oil phase. The oil phase was rectified under reduced pressure to obtain 602 g of phenyldihydropyran with a content of 96%...
Embodiment 2
[0035] Repeat the steps A to B above to recover 604g of phenyldihydropyran with a content of 95% and recover 505g of tert-butylbenzene. The recovered tert-butylbenzene contains a small amount of benzaldehyde and prenol.
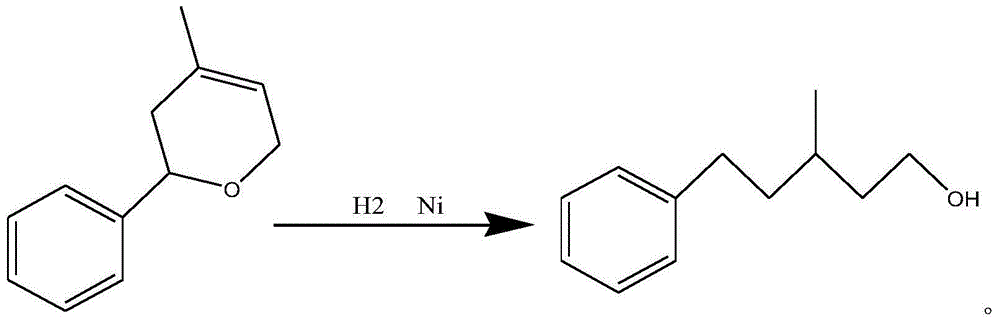
[0036] Step C: Put 604g of recovered phenyldihydropyran, 14g of recovered Raney nickel and newly added 3g of Raney nickel into a 1000mL hydrogenation kettle to raise the temperature to 130-140°C, pressure 0.6-1MPa, and start hydrogenation , after hydrogenation to 3mol, the reaction time is 2.5h, the hydrogenation is about 40% of the theoretical hydrogenation amount, stop hydrogenation, cool down, and filter.
[0037] Step D: Put the hydrogenation filtered clear liquid back into the hydrogenation kettle, put in the first batch of recycled palladium carbon and 1g of new 5% palladium carbon, and heat up to 130-140°C, pressure 0.5-0.6MPa, and start hydrogenation. The hydrogenation continued for 4.5 hours until no more hydrogen was absorbed, the reaction was stopp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com