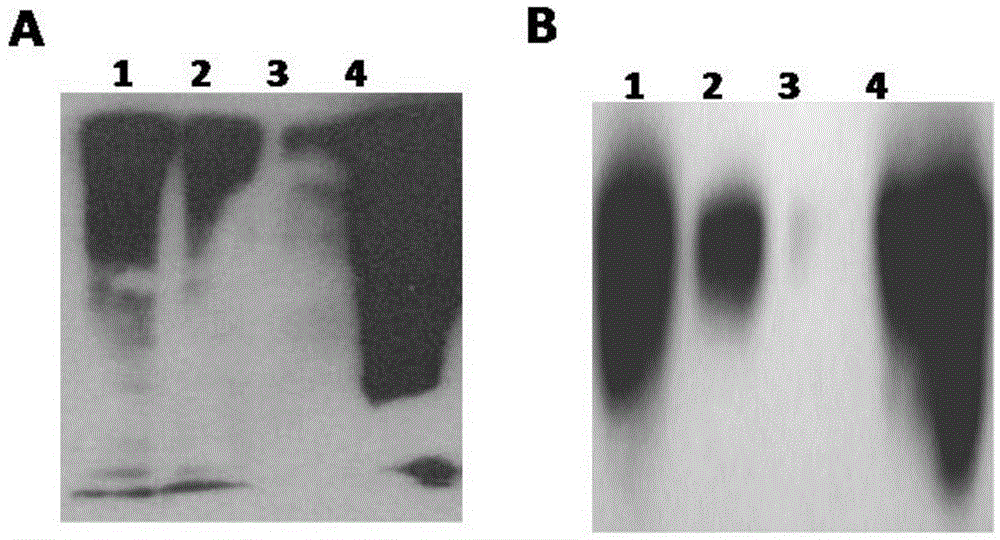
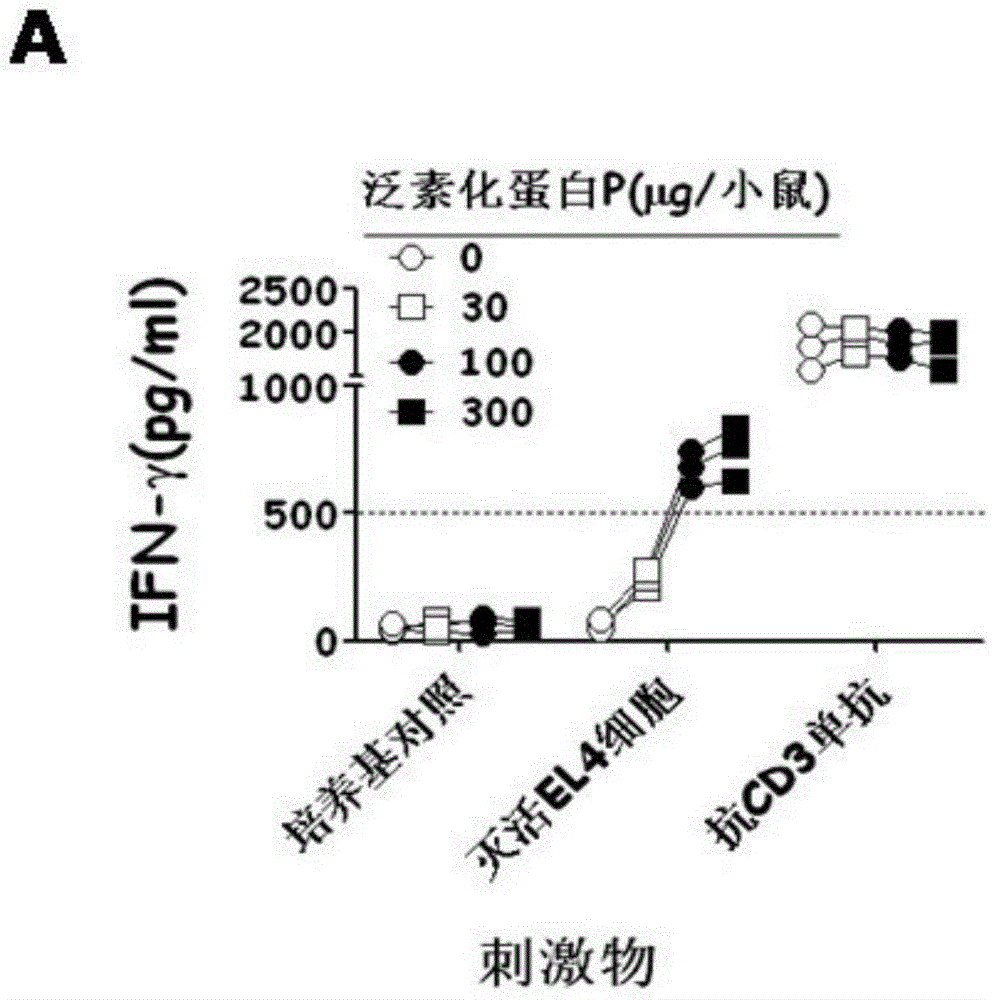
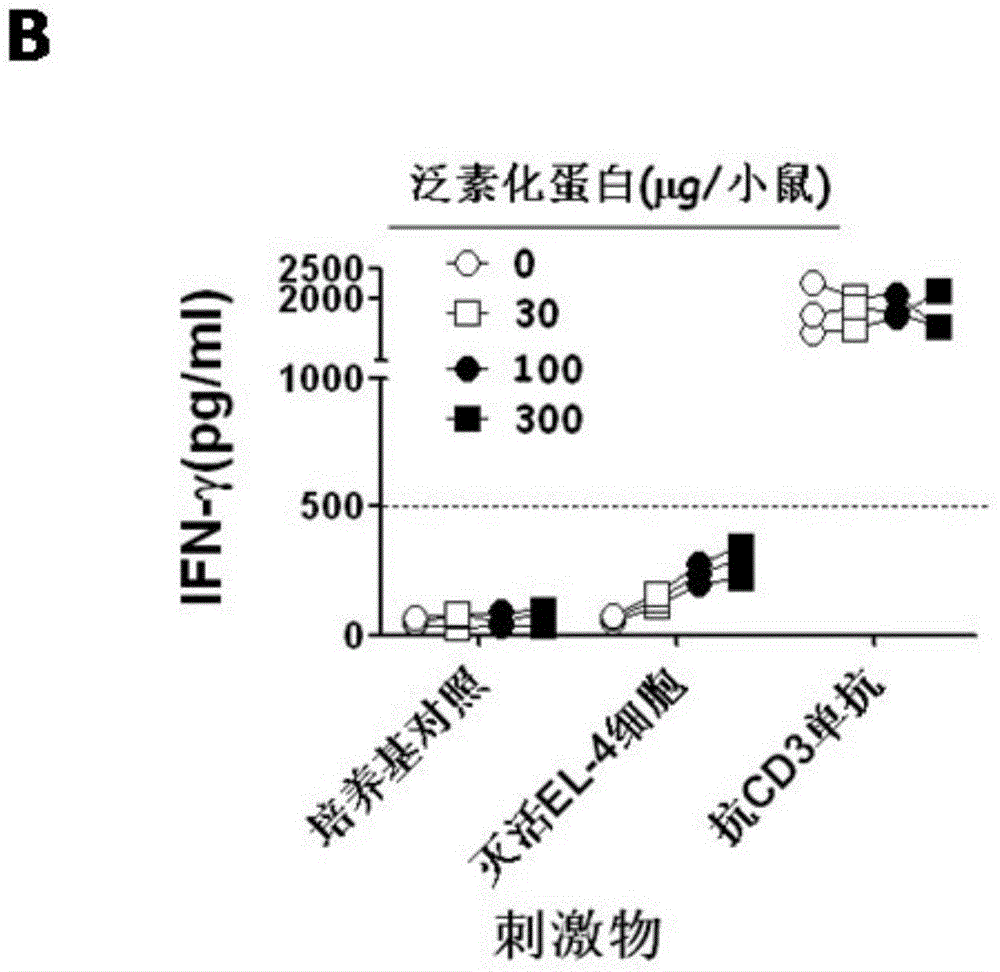
Anti-tumor ubiquitinated protein and extraction method and application thereof
A technology of ubiquitinated protein and extraction method, which is applied in the fields of antitumor drugs, chemical instruments and methods, and pharmaceutical formulations, and can solve the asymmetry between ubiquitinated short-lived proteins and long-lived proteins, poor anti-tumor effect of tumor vaccines, Ubiquitinated short-lived protein degrades quickly and other problems, to achieve the effect of inhibiting tumor growth, overcoming asymmetry and improving the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 1. Extraction method of Vx3(A7) protein:
[0074] (1) Transform the pUbiG101-Vx3(A7)-eGFP plasmid into Escherichia coli E.coli(DH5-α);
[0075] (2) pick a single clone of the Escherichia coli obtained in step (1), culture it in LB (Luria-Bertani) medium, and perform double enzyme digestion identification;
[0076] (3) Expand the culture of the bacteria identified in step (2), and add IPTG (Isopropylβ-D-1-thiogalactopyranoside, isopropyl-β-D-thiogalactopyranoside) when the absorbance value of the bacteria solution is 0.5 ) for 16 hours of induction, high-speed centrifugation at 8000 rpm for 15 minutes, and discarding the supernatant;
[0077] (4) Use Bing buffer (50mM NaH 2 PO 4 , 300mM NaCl, 25mM imidazole, pH8.0) resuspended, added lysozyme to react on ice for 30min;
[0078] (5) collect the supernatant that step (4) obtains, join it in the nickel ion affinity chromatography, after treating to combine with column material, wash buffer (50mM NaH 2 PO4, 300mM NaCl, ...
Embodiment 2
[0086] 1. Extraction method of Vx3(A7) protein:
[0087] (1) Transform the pUbiG101-Vx3(A7)-eGFP plasmid into Escherichia coli E.coli(DH5-α);
[0088] (2) pick a single clone of the Escherichia coli obtained in step (1), culture it in LB (Luria-Bertani) medium, and perform double enzyme digestion identification;
[0089] (3) Expand the culture of the bacteria identified in step (2), and add IPTG (Isopropylβ-D-1-thiogalactopyranoside, isopropyl-β-D-thiogalactopyranoside) when the absorbance value of the bacteria solution is 0.5 ) for 16 hours of induction, high-speed centrifugation at 8000 rpm for 15 minutes, and discarding the supernatant;
[0090] (4) Use Bing buffer (50mM NaH 2 PO 4 , 300mM NaCl, 25mM imidazole, pH8.0) resuspended, added lysozyme to react on ice for 30min;
[0091] (5) collect the supernatant that step (4) obtains, join it in the nickel ion affinity chromatography, after treating to combine with column material, wash buffer (50mM NaH 2 PO4, 300mM NaCl, 15...
Embodiment 3
[0098] Embodiment 3: (tumor cells are EL4 cells)
[0099] 1. Extraction method of Vx3(A7) protein:
[0100] (1) Transform the pUbiG101-Vx3(A7)-eGFP plasmid into Escherichia coli E.coli(DH5-α);
[0101] (2) pick a single clone of the Escherichia coli obtained in step (1), culture it in LB (Luria-Bertani) medium, and perform double enzyme digestion identification;
[0102] (3) Expand the culture of the bacteria identified in step (2), and add IPTG (Isopropylβ-D-1-thiogalactopyranoside, isopropyl-β-D-thiogalactopyranoside) when the absorbance value of the bacteria solution is 0.5 ) for 16 hours of induction, high-speed centrifugation at 8000 rpm for 15 minutes, and discarding the supernatant;
[0103] (4) Use Bing buffer (50mM NaH 2 PO 4 , 300mM NaCl, 25mM imidazole, pH8.0) resuspended, added lysozyme to react on ice for 30min;
[0104] (5) collect the supernatant that step (4) obtains, join it in the nickel ion affinity chromatography, after treating to combine with column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com