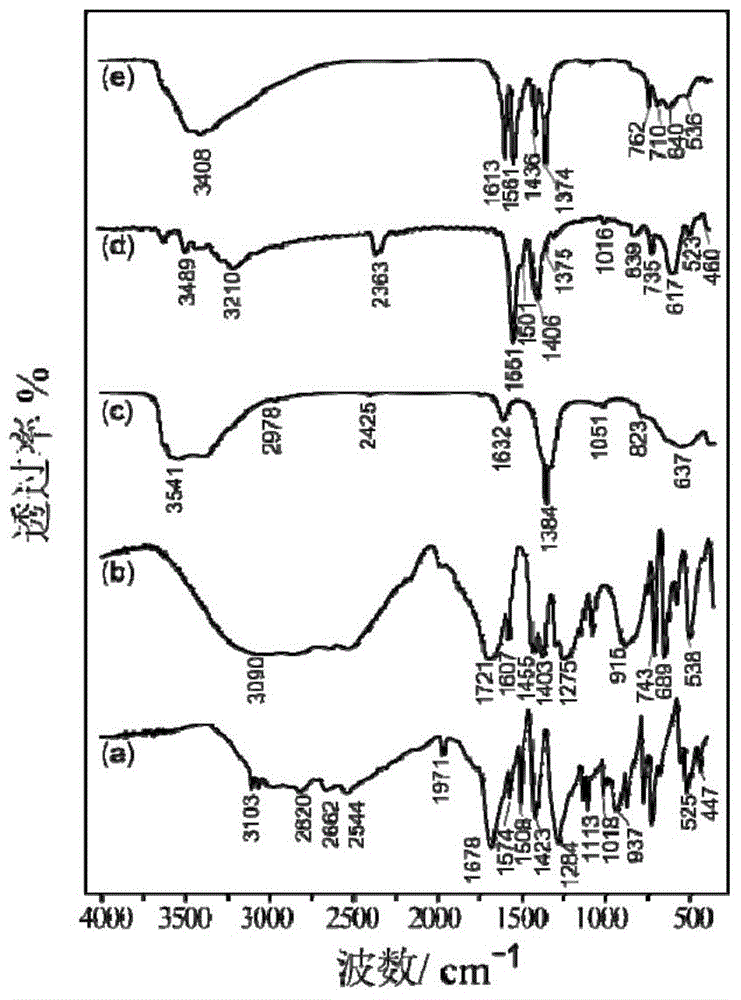
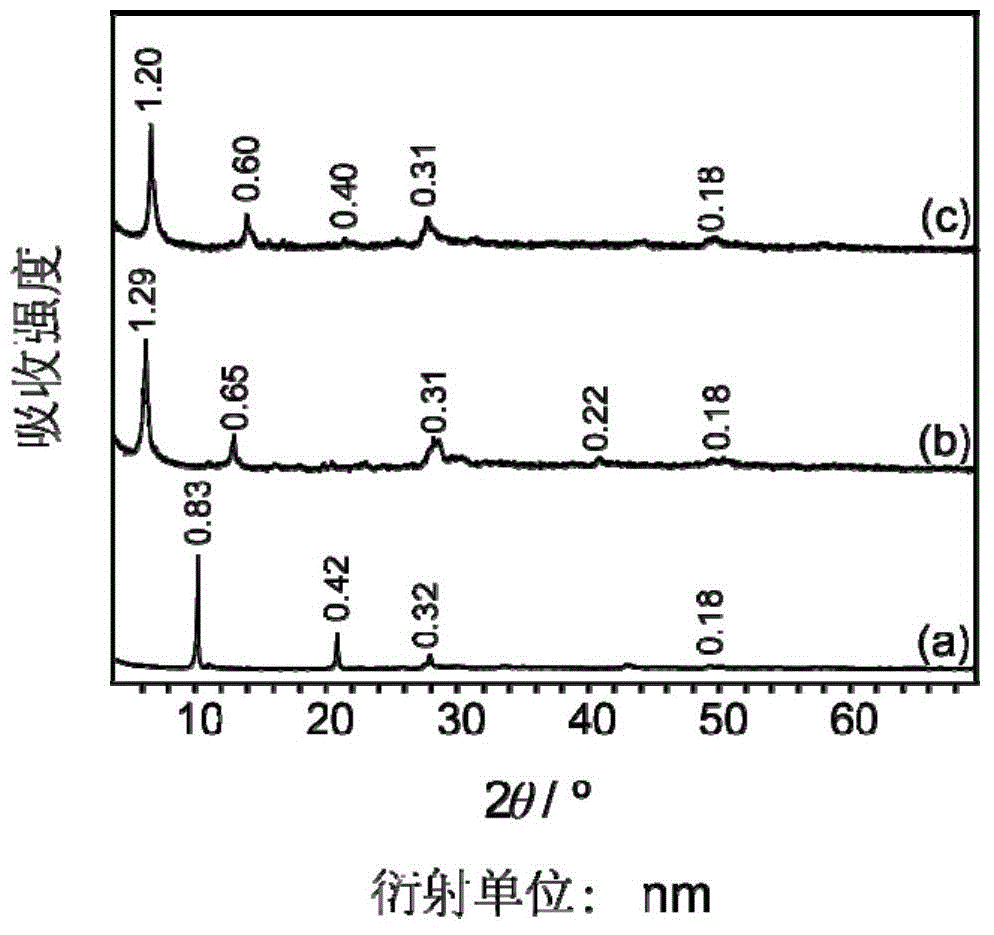
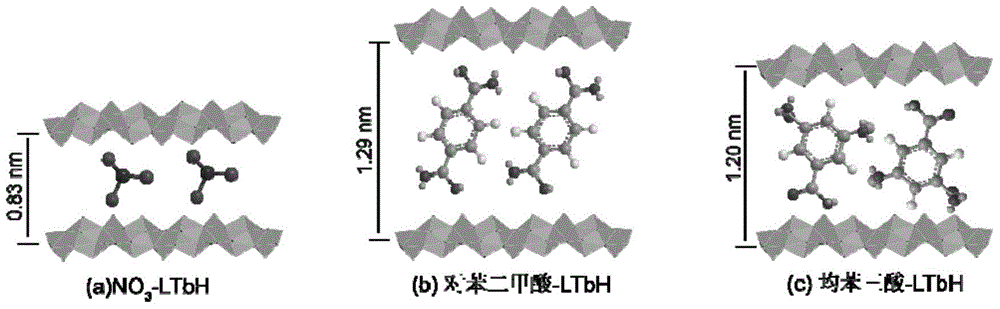
Trimesic acid-LTbH complex and synthesis method thereof
A technique for the synthesis of trimesic acid and its synthesis method, which is applied in the field of trimesic acid-LTbH complex and its synthesis, which can solve the problems of affecting the luminous intensity, fluorescence quenching, and failure to meet the requirements, and achieve the effect of improving the luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1), using the uniform precipitation method to synthesize NO 3 -LTbH precursor
[0042] 0.453g (1mmol) Tb (NO 3 ) 3 ·6H 2 O, 1.10g (13mmol) NaNO 3 , 0.14g (1mmol) of hexamethylenetetramine (HMT) was dissolved in 80ml of degassed water to obtain an aqueous solution, and then hydrothermally reacted at 70°C for 16 hours. After the reaction, the obtained product was filtered by suction, washed with deionized water, and then filtered by suction, repeated 3 times, and dried in vacuum for 24 hours to obtain white powdery NO 3 - LTbH 0.251 g. to NO 3 Based on -LTbH, the yield was 96%.
[0043] (2), using ion exchange method to synthesize terephthalic acid-LTbH complex
[0044]0.166 g (1 mmol) of terephthalic acid and 0.060 g (1.5 mmol) of NaOH were added into 5 mL of deionized water to dissolve them completely to obtain a clear aqueous solution of sodium terephthalate salt.
[0045] 0.033g NO 3 -LTbH was dispersed in 75ml of deionized water, mixed with the above-prepa...
Embodiment 2
[0050] (1), using the uniform precipitation method to synthesize NO 3 -LTbH precursor
[0051] 0.453g (1mmol) Tb (NO 3 ) 3 ·6H 2 O, 1.650g (19.5mmol) NaNO 3 , 0.21g (1.5mmol) of hexamethylenetetramine was dissolved in 120ml of degassed water to obtain an aqueous solution, and then hydrothermally reacted at 90°C for 12 hours. After the reaction, the obtained product was filtered by suction, washed with deionized water and then filtered by suction, repeated 3 times, and then vacuum-dried for 24 hours to obtain white powdery NO 3 -LTbH 0.257g. to NO 3 Based on -LTbH, the yield was 98%.
[0052] (2), using ion exchange method to synthesize terephthalic acid-LTbH complex
[0053] 0.166 g (1 mmol) of terephthalic acid and 0.080 g (2 mmol) of NaOH were added into 5 mL of deionized water to dissolve them completely to obtain a clear aqueous solution of sodium terephthalate salt.
[0054] 0.087g NO 3 -LTbH was dispersed in 100 ml of deionized water, mixed with the aqueous sol...
Embodiment 3
[0059] (1), using the uniform precipitation method to synthesize NO 3 -LTbH precursor
[0060] 0.453g (1mmol) Tb (NO 3 ) 3 ·6H 2 O, 2.20g (26mmol) NaNO 3 , 0.28g (2mmol) of hexamethylenetetramine was dissolved in 160ml of degassed water to obtain an aqueous solution, which was hydrothermally reacted at 100°C for 18 hours. After the reaction, the obtained product was filtered by suction, washed with deionized water, and then filtered by suction, repeated 3 times, and dried in vacuum for 24 hours to obtain white powdery NO 3 - LTbH 0.258 g. to NO 3 Based on -LTbH, the yield was (98.8%).
[0061] (2), using ion exchange method to synthesize terephthalic acid-LTbH complex
[0062] 0.166 g (1 mmol) of terephthalic acid and 0.072 g (1.8 mmol) of NaOH were added into 5 mL of deionized water to dissolve them completely to obtain a clear aqueous solution of sodium terephthalate salt.
[0063] 0.118g NO 3 -LTbH was dispersed in 150 ml of deionized water, mixed with the aqueous...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap