Iron-based catalyst and preparation method thereof
A catalyst and iron-based technology, applied in the field of iron-based catalysts and their preparation, can solve problems such as unfavorable cost saving, large-scale development and application, high raw material cost, complicated preparation process or used equipment, etc. Activity, the effect of increasing coal conversion and oil production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
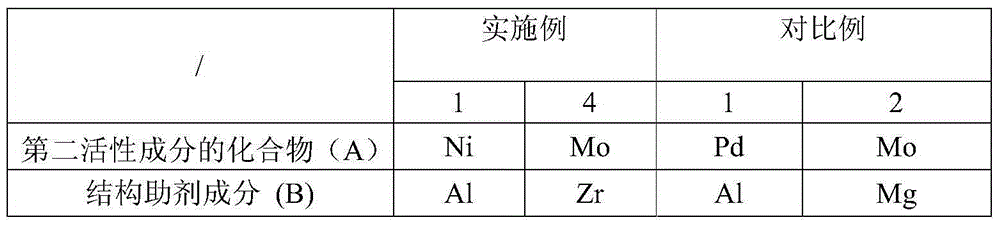
[0026] In another typical embodiment of the present invention, a method for preparing an iron-based catalyst is provided. The preparation method includes: step S1, preparing a FeOOH filter cake, the filter cake contains a structural aid component and an optional part of the second Active ingredient; step S2, making FeOOH filter cake into powdered FeOOH; step S3, loading all or remaining second active ingredients on powdery FeOOH to obtain iron-based catalyst; wherein, the second active ingredient includes nickel, molybdenum, cobalt and one or more elements of tungsten; the structural aid component includes one or more elements of silicon, aluminum and zirconium.
[0027] In the above-mentioned preparation method of the present invention, by adopting the structural aid component containing the above-mentioned elements to form a FeOOH filter cake with the first active component Fe, and according to the difference of the element type contained in the second active component, optio...
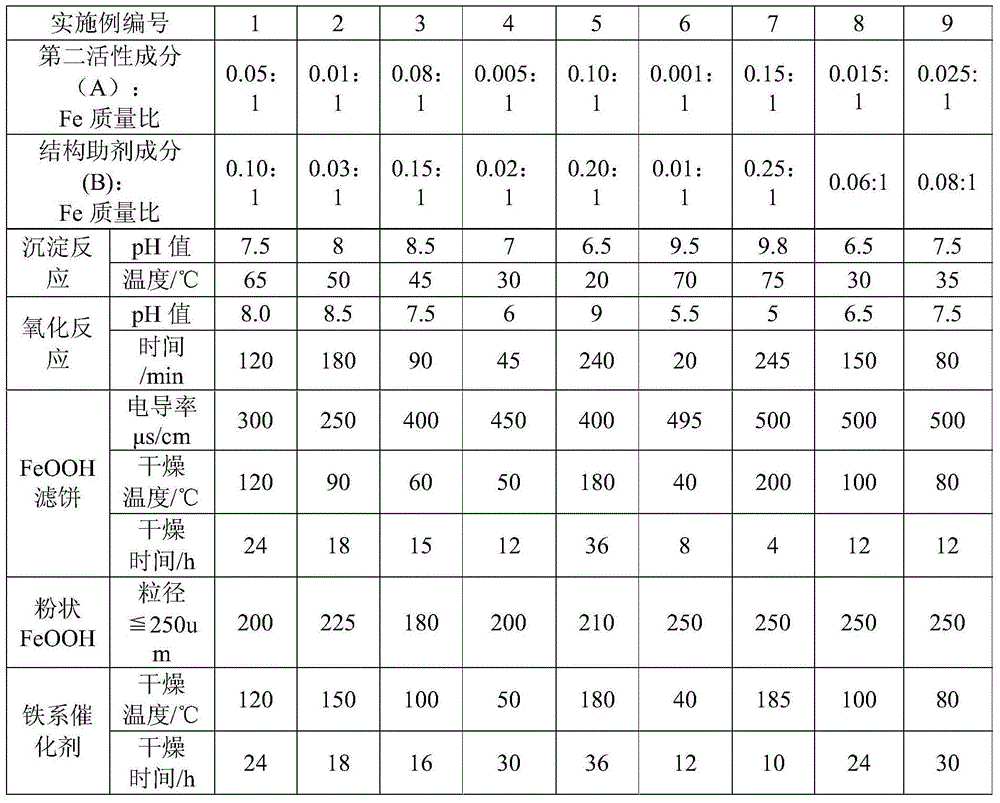
Embodiment 1
[0041] Weigh 51.16g FeCl respectively 2 4H 2 O, 1.86g Ni(NO 3 ) 2 ·6H 2 O, 20.83g Al(NO 3 ) 3 9H 2 O was dissolved in 600g deionized water together to obtain a mixed solution containing ferrous, nickel and aluminum; weigh 50g Na 2 CO 3 Add to 500g deionized water to form a sodium carbonate solution;
[0042] Using a peristaltic pump, the above-mentioned mixed solution containing ferrous, nickel, aluminum and sodium carbonate solution flow together to form a reaction solution, which is added to a 5L reactor with 500g of cushion water for precipitation reaction. The reaction temperature is controlled to be 65°C, and the feed time is For 45 minutes, control the feed rate of ammonia water to maintain the reaction pH value at 7.5;
[0043] After the precipitation reaction was completed, feed into the air to carry out the oxidation reaction. The oxidation reaction time was 120 minutes. During this period, the reaction pH value was maintained at 8.0 by controlling the sodium...
Embodiment 2
[0047] Weigh 51.16g FeCl 2 4H 2 O and 0.37g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 600g of deionized water to obtain a mixed solution containing ferrous and nickel; at the same time, 40g of concentrated ammonia water with a concentration of 27wt% was weighed and added to 500g of deionized water to form a dilute ammonia solution, and then 1.96g of Na 2 SiO 3 Dissolved in dilute ammonia solution;
[0048] Using a peristaltic pump, the above-mentioned mixed solution containing ferrous and nickel and the ammonia solution containing silicon flow together to form a reaction solution, which is added to a 5L reactor with 500g of cushion water for precipitation reaction. The reaction temperature is controlled to be 50°C, and the feed time is 35 minutes, control the feed rate of ammonia water to maintain the reaction pH value as 8.0;
[0049] After the precipitation reaction was completed, air was introduced to carry out the oxidation reaction. The oxidation reaction time was 180 mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com