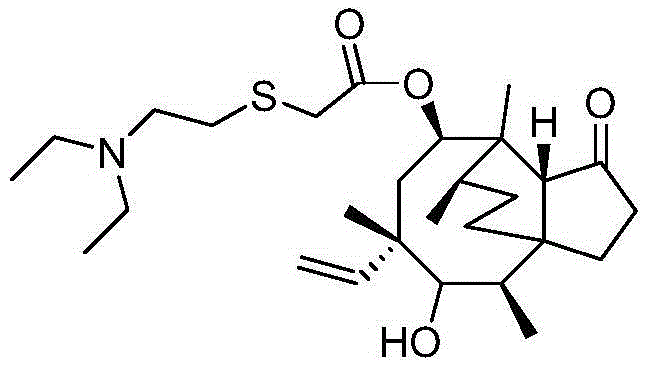
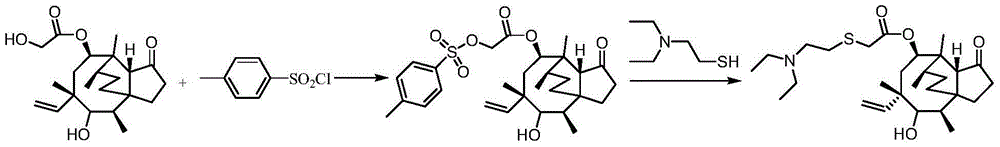
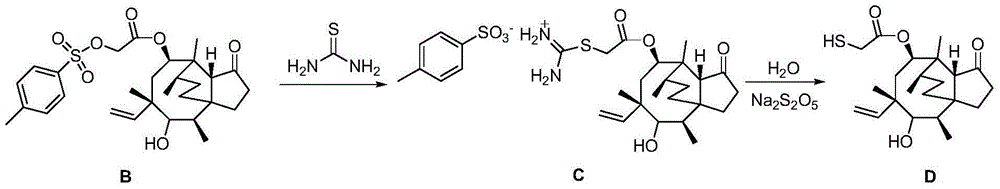
Synthesis method of tiamulin
A tiamulin and synthesis method technology, applied in the field of chemical synthesis of veterinary raw materials, can solve the problems of difficult diethylaminoethanethiol, high price, bad smell, etc., and achieve the effect of simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Add 31.97g (0.42mol) thiourea to the methyl isobutyl ketone solution containing 213.07g (0.4mol) pleuromutilin p-toluenesulfonate, stir and react at 60°C for 1.5 hours, then add 30.42g (0.16mol) of sodium metabisulfite, 120g of distilled water, reflux at 90°C for 1 hour, separate the layers, and remove the water layer to obtain a methyl isobutyl ketone solution of pleuromutilin.
[0024] 2. Add 92.93g (0.54mol) 2-diethylamino-1-chloroethane hydrochloride, 21.60g (0.54mol) to the methyl isobutyl ketone solution of pleuromutilin obtained in step 1 NaOH and 240 g of distilled water were stirred at 60° C. for 2 hours, the water layer was removed, and the organic layer was concentrated to dryness under reduced pressure to obtain 170.24 g of tiamulin with a total yield of 86.2%.
Embodiment 2
[0026] In step 2 of embodiment 1, the 2-diethylamino-1-chloroethane hydrochloride used is replaced with equimolar 2-diethylamino-1-bromoethane hydrobromide, other steps and implementation Same as Example 1, 162.74 g of tiamulin was obtained, and the total yield was 82.4%.
Embodiment 3
[0028] In step 2 of Example 1, the 2-diethylamino-1-chloroethane hydrochloride used is replaced with equimolar 2-diethylamino-1-chloroethane bisulfate, and other steps are the same as in the examples 1 was the same, and 159.89 g of tiamulin was obtained, with a total yield of 81.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com