2-oxo-1-pyrrolidine chiral derivative preparation method
A technology for pyrrolidine and derivatives, which is applied in the field of preparation of 2-oxo-1-pyrrolidine chiral derivatives, can solve the problems of difficult mass production, high price, high production cost, etc., to facilitate industrial production and reduce production The effect of high cost and product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
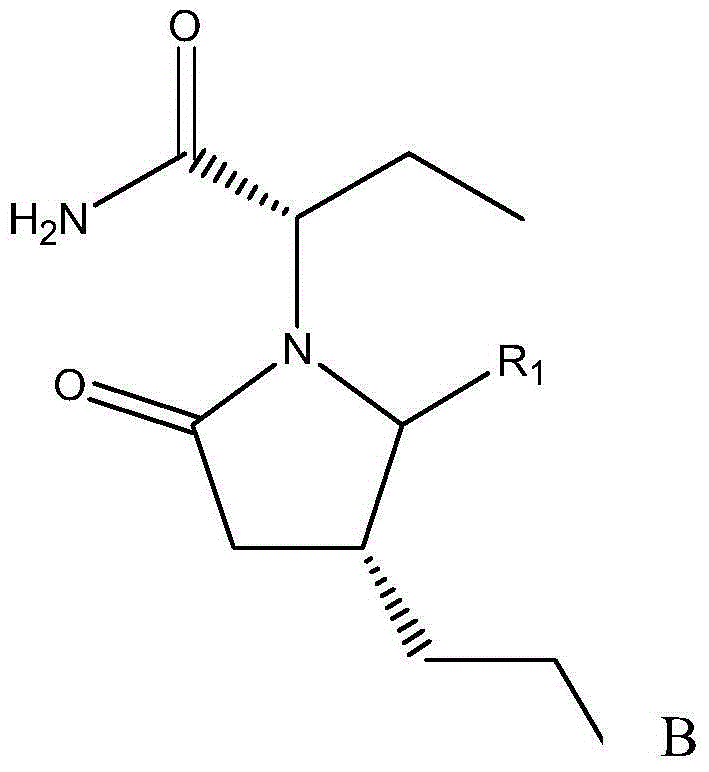
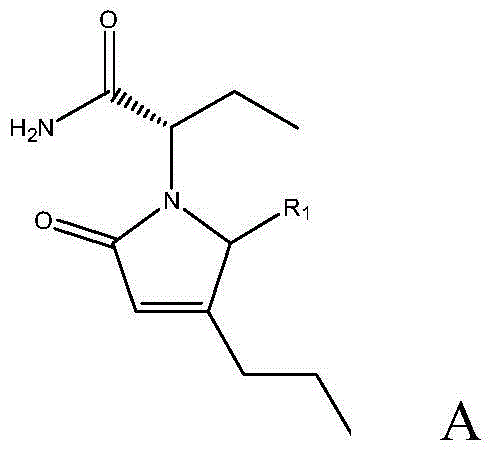
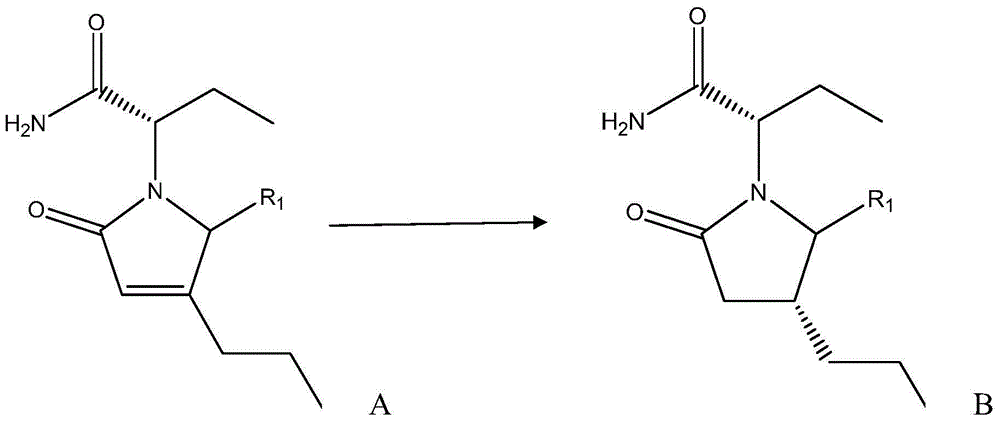
[0030] The preparation method of (2S)-2-((4R)-2-oxo-4-n-propyl-1-pyrrolidinyl)butyramide comprises the following steps: adding 430mg to the reaction vessel under the protection of nitrogen Cuprous chloride, 420mg sodium tert-butoxide, 120mg(R)-DTBM-SEGPHOS and 240ml toluene, stirred for 20min; then adjusted the temperature to -40°C, and added 60g of the reaction raw material, that is, R in formula A 1 Substance that is hydrogen—(2S)-(2-oxo-4-propyl-2,5-dihydro-1-pyrrolyl)butanamide; then, add 40ml of polymethylhydrogensiloxane to the reaction vessel alkane (that is, 0.67mol of polymethylhydrogensiloxane), and then react at a constant temperature at -40°C for 24h; after the reaction, adjust the temperature to 0°C, and then add 100ml of saturated sodium bicarbonate solution and 150ml of ether, Then stir for 10 h; then, use diethyl ether to extract the reaction material twice, combine the organic layers obtained by the two extractions, then dry, filter and concentrate the organic...
Embodiment 2
[0032](2S)-2-((4R)-2-oxo-4-n-propyl-5-carboxy-1-pyrrolidinyl) the preparation method of butanamide, it comprises the following steps: under the protection of nitrogen, reaction Add 430 mg cuprous chloride, 420 mg sodium tert-butoxide, 120 mg (R)-DTBM-SEGPHOS and 240 ml toluene into the container, stir for 20 min; then adjust the temperature to -40 °C, and add 73 g of reaction raw materials, that is, R1 in molecular formula A is Carboxylic substance—(2S)-(2-oxo-4-propyl-2,5-dihydro-5-carboxy-1-pyrrolyl)butanamide; then, add 40ml of polymethyl Hydrogen siloxane (that is, 0.67mol of polymethyl hydrogen siloxane), and then react at a constant temperature at -40°C for 24h; after the reaction, adjust the temperature to 0°C, and then add 100ml of saturated sodium bicarbonate solution and 150ml of diethyl ether, and then stirred for 10h; then, the reaction material was extracted twice with diethyl ether, and the organic layers obtained by the two extractions were combined, and then th...
Embodiment 3
[0034] The preparation method of (2S)-2-((4R)-2-oxo-4-n-propyl-5-sulfonic acid group-1-pyrrolidinyl)butyramide comprises the steps of: under the protection of nitrogen Add cuprous chloride, potassium tert-butoxide, (R)-DTBM-SEGPHOS and 160ml isopropanol in reaction vessel, stir 15min, the molar weight of described cuprous chloride is 0.1% of reaction raw material molar weight, so The molar weight of potassium tert-butoxide is 0.1% of the molar weight of the reaction raw materials, and the molar weight of the (R)-DTBM-SEGPHOS is 0.1% of the molar weight of the reaction raw materials; Raw materials, that is, substances in which R1 is a sulfonic acid group in molecular formula A; then, add formic acid to the reaction vessel, and the molar ratio of the formic acid to the reaction raw materials is 5:1; then react at a constant temperature at -80°C for 48h; react After the end, adjust the temperature to -20°C, then add 80ml of saturated sodium carbonate solution and 80ml of methyl t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com