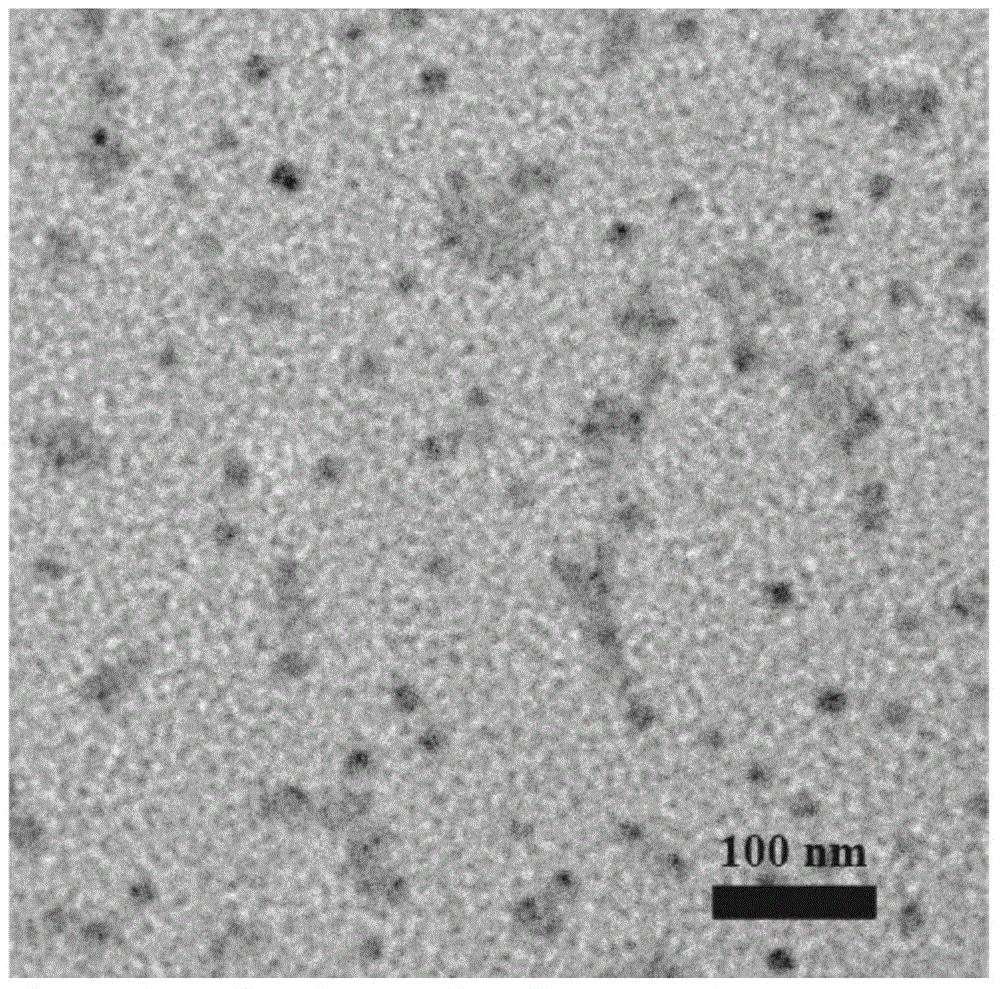
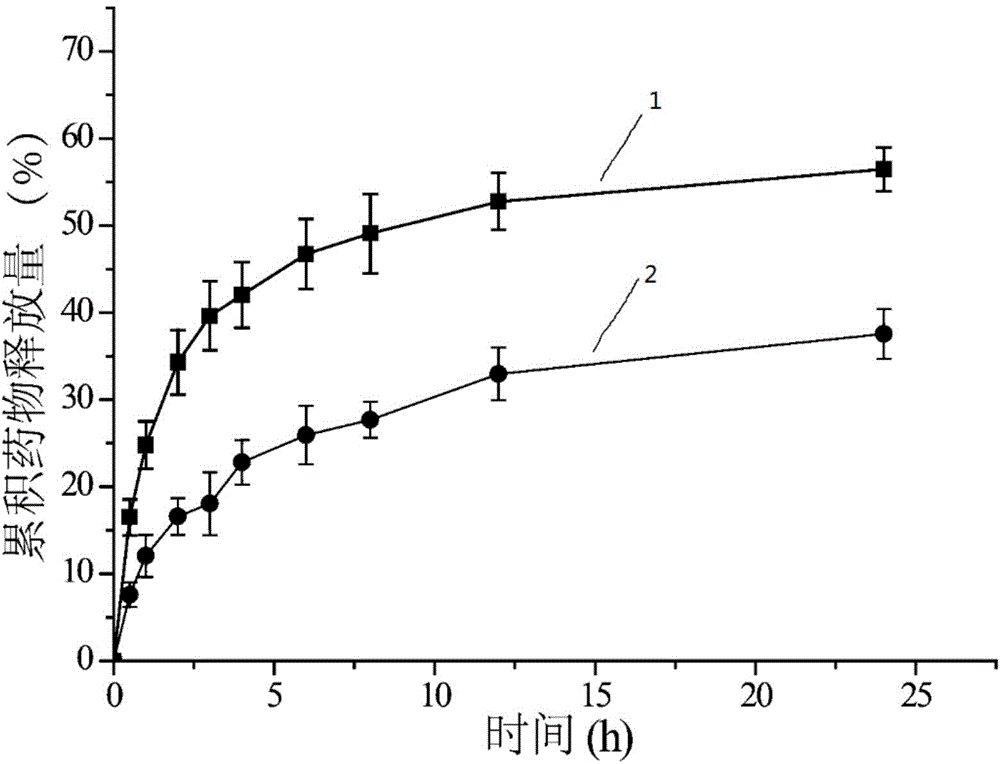
Preparation of cross-linked nano-micelle with redox sensitive performance
A nano-micelle and sensitive technology, which is applied in the field of chemically cross-linked and redox-sensitive micelles and its preparation, can solve the problems of cumbersome synthesis process and low modification, and achieve easy products, mild reaction conditions, and easy The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 13mg of cumyl dithiobenzoate, 1.06g of glycidyl methacrylate and 3mg of azobisisobutyronitrile in 1.5mL of tetrahydrofuran, degas three times through continuous freezing and thawing, and place in a 70°C refrigerator after being protected by argon. Polymerization in an oil bath for 8 hours. After the end, the reaction was quenched with liquid nitrogen to stop the reaction, and the method of dissolving tetrahydrofuran by precipitation with n-hexane was used to obtain poly(glycidyl methacrylate) macromolecular chain transfer agent after repeated purification 3 times;
[0022] Dissolve 0.24g of poly(glycidyl methacrylate) macromolecular chain transfer agent, 0.16g of polyethylene glycol methacrylate and 3mg of azobisisobutyronitrile in 1mL of tetrahydrofuran, and degas five times through continuous freezing and thawing. After being filled with argon for protection, it was placed in an oil bath at 65°C for a polymerization reaction for 12 hours. After the end, the ...
Embodiment 2
[0025] Dissolve 14mg of 4-cyano-4-(phenylthioformylthio)pentanoic acid, 1.42g of 4-(formyl)phenyl methacrylate and 3mg of azobisisobutyronitrile in 2.5mL of tetrahydrofuran, and After freeze-thawing and degassing for 4 times, it was protected by argon and placed in an oil bath at 80°C for 24 hours of polymerization reaction. After the end, the reaction was quenched with liquid nitrogen to stop the reaction, and the method for dissolving tetrahydrofuran by precipitation with n-hexane was used to obtain poly(4-(formyl)phenyl methacrylate) macromolecular chain transfer agent after repeated purification for 5 times;
[0026] Dissolve 0.48g of poly(4-(formyl)phenyl methacrylate) macromolecular chain transfer agent, 0.8g of polyethylene glycol methacrylate and 3mg of azobisisobutyronitrile in 1mL of tetrahydrofuran. Gas 3 times, filled with argon protection, and placed in an oil bath at 75°C for 36 hours of polymerization. After the end, the liquid nitrogen was quenched to stop the...
Embodiment 3
[0029] Dissolve 14mg of 4-cyano-4-(phenylthioformylthio)pentanoic acid, 0.95g of 4-(formyl)phenyl methacrylate and 3mg of azobisisobutyronitrile in 2mL of tetrahydrofuran, and freeze Melting and degassing for 5 times, filling with argon for protection, and placing in an oil bath at 65°C for 48 hours of polymerization. After the end, the reaction was quenched with liquid nitrogen to stop the reaction, and the method for dissolving tetrahydrofuran by precipitation with n-hexane was used to obtain poly(4-(formyl)phenyl methacrylate) macromolecular chain transfer agent after repeated purification for 5 times;
[0030] Dissolve 0.32g of poly(4-(formyl)phenyl methacrylate) macromolecular chain transfer agent, 0.15g of polyethylene glycol methacrylate and 3mg of azobisisobutyronitrile in 1.5mL of tetrahydrofuran, and freeze-thaw continuously After degassing for 3 times, protect it with argon and place it in an oil bath at 80°C for 24 hours of polymerization reaction. After the end, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com