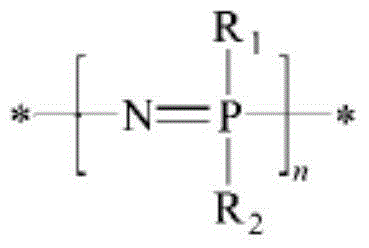
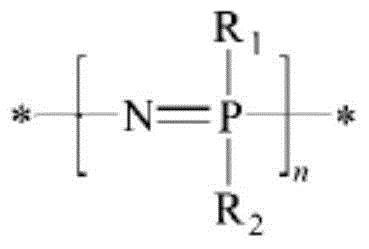
Preparation method of self-crosslinked phosphazene elastomer
A self-crosslinking and elastomer technology, applied in the field of polyphosphazene, can solve the problems affecting the thermal stability of cross-linked phosphazene elastomer products, cross-linked polyphosphazene elastomers, and cross-linking conditions by adding cross-linking agents, etc. The problem is that the preparation method and the cross-linking method are simple, the difficulty of cross-linking and the cost of cross-linking are reduced, and the cost is low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under the protection of nitrogen, add ethylene glycol to the flask connected with the condenser tube, the substance ratio of alcohol to sodium hydride is 1.5:1, and the solvent tetrahydrofuran, and add sodium hydride to the flask at room temperature until the sodium hydride is completely The reaction obtains ethylene glycol monosodium salt solution; under nitrogen protection, add n-butanol to a flask connected with a condenser, the mol ratio of alcohol to sodium hydride is 1.5:1, and solvent tetrahydrofuran, at room temperature the sodium hydride Add in the flask until sodium hydride reacts completely to obtain sodium n-butoxide solution; under nitrogen protection, add phenol to the flask connected with the condenser tube, the molar ratio of phenol to sodium hydride is 1.5:1, and solvent tetrahydrofuran, at room temperature Next, add sodium hydride into the flask until the sodium hydride reacts completely to obtain a sodium phenoxide solution; take a tetrahydrofuran solu...
Embodiment 2
[0025] Under the protection of nitrogen, add ethylene glycol to the flask connected with the condenser tube, the substance ratio of alcohol to sodium hydride is 1.5:1, and the solvent tetrahydrofuran, and add sodium hydride to the flask at room temperature until the sodium hydride is completely The reaction obtains ethylene glycol monosodium salt solution; under nitrogen protection, add n-butanol to a flask connected with a condenser, the mol ratio of alcohol to sodium hydride is 1.5:1, and solvent tetrahydrofuran, at room temperature the sodium hydride Add in the flask until sodium hydride reacts completely to obtain sodium n-butoxide solution; under nitrogen protection, add phenol to the flask connected with the condenser tube, the molar ratio of phenol to sodium hydride is 1.5:1, and solvent tetrahydrofuran, at room temperature Next, add sodium hydride into the flask until the sodium hydride reacts completely to obtain a sodium phenoxide solution; take a tetrahydrofuran solu...
Embodiment 3
[0027] Under the protection of nitrogen, add ethylene glycol to the flask connected with the condenser tube, the substance ratio of alcohol to sodium hydride is 1.5:1, and the solvent tetrahydrofuran, and add sodium hydride to the flask at room temperature until the sodium hydride is completely The reaction obtains ethylene glycol monosodium salt solution; under the protection of nitrogen, add trifluoroethanol to the flask connected with the condenser, the mol ratio of alcohol to sodium hydride is 1.5:1, and solvent tetrahydrofuran, at room temperature the sodium hydride Add in the flask until sodium hydride reacts completely to obtain sodium trifluoroethoxide solution; under nitrogen protection, add phenol to the flask connected with the condenser tube, the molar ratio of phenol to sodium hydride is 1.5:1, and solvent tetrahydrofuran, at room temperature Next, add sodium hydride into the flask until the sodium hydride reacts completely to obtain a sodium phenoxide solution; ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com