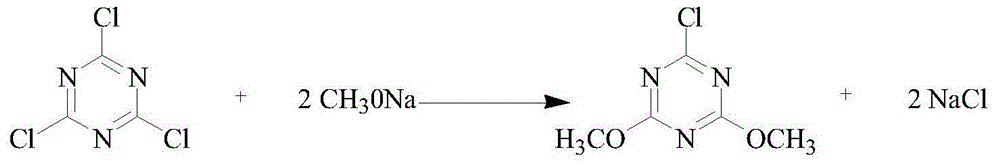
Preparation method of 2-chloro-4,6-dimethoxy-1,3,5-triazine
A technology of dimethoxyl and dimethylformamide, applied in the direction of organic chemistry, can solve the problems of troublesome post-processing, high content of monosubstituted triazine, high content of monosubstituted triazine and trisubstituted triazine, and achieve reduction The effect of production cost, improvement of product purity, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Preparation of 2-chloro-4,6-dimethoxy-1,3,5-triazine crude product: first add 500kgN,N-dimethylformamide and 369kg cyanuric chloride to the reactor, After the cyanuric chloride is completely dissolved, the reaction kettle is cooled to 5-10°C, and then 245kg of sodium methoxide solid is added. After the addition, it is reacted at room temperature for 2 hours, and then heated to reflux for 2.5 hours. After the reaction, add a large amount of water to the reaction solution , filtered, the obtained solid phase was washed with water, suction filtered, and finally dried to obtain 345kg of crude 2-chloro-4,6-dimethoxy-1,3,5-triazine;
[0019] (2) Purification of crude 2-chloro-4,6-dimethoxy-1,3,5-triazine: Dissolve the obtained crude product in 750kg of heptane for recrystallization, filter, and recover 705kg of heptane from the liquid phase by distillation alkane, and solid-phase drying to obtain 316kg of 2-chloro-4,6-dimethoxy-1,3,5-triazine with a yield of 91.25%.
Embodiment 2
[0021] (1) Preparation of 2-chloro-4,6-dimethoxy-1,3,5-triazine crude product: first add 500kgN,N-dimethylformamide and 369kg cyanuric chloride to the reactor, After the cyanuric chloride is completely dissolved, the temperature of the reaction kettle is lowered to 5-10°C, and then 260kg of sodium methoxide solid is added. After the addition, it is reacted at room temperature for 2 hours, and then the temperature is raised to reflux for 2.5 hours. After the reaction, a large amount of water is added to the reaction solution , filtered, the obtained solid phase was washed with water, suction filtered, and finally dried to obtain 351kg of crude 2-chloro-4,6-dimethoxy-1,3,5-triazine;
[0022] (2) Purification of crude 2-chloro-4,6-dimethoxy-1,3,5-triazine: dissolve the obtained crude product in 750kg heptane for recrystallization, filter, and recover 700kg heptane from the liquid phase by distillation alkane, and solid-phase drying to obtain 313kg of 2-chloro-4,6-dimethoxy-1,3,5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com