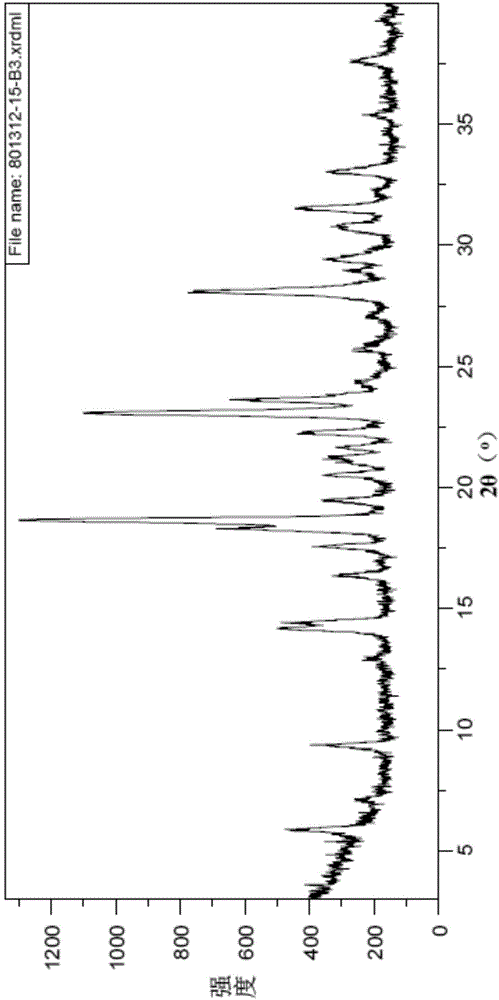
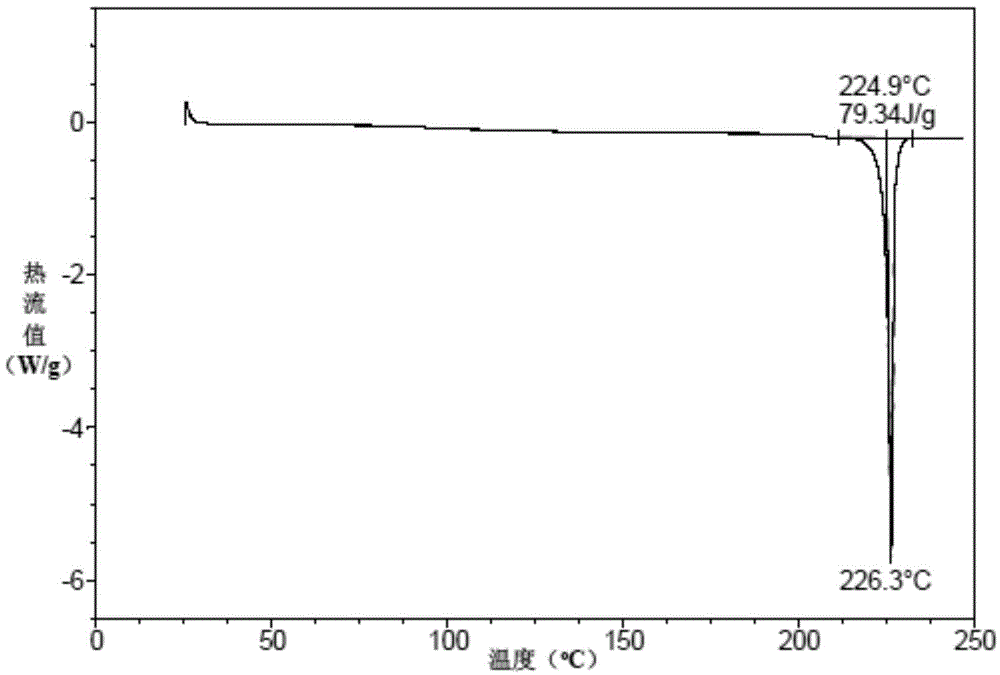
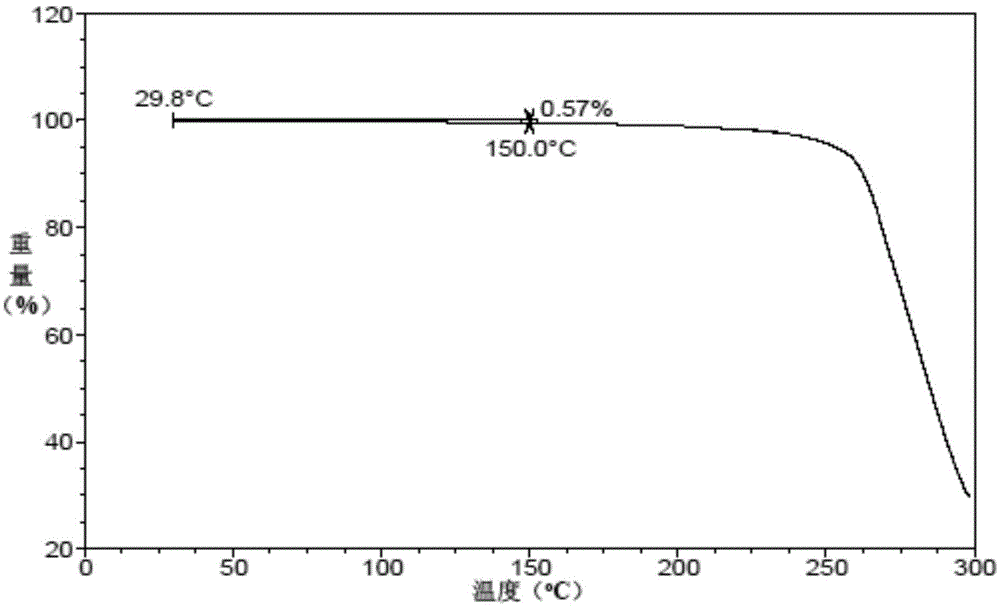
Vortioxetine hydrobromide crystal preparation method
A technology of vortioxetine hydrobromide and vortioxetine, which is applied in the field of medicine and can solve the problems of great harm to the human body and cumbersome operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Preparation of vortioxetine hydrobromide crystal
[0040] Dissolve 10 g of vortioxetine free base in 350 ml of ethyl acetate, stir to dissolve at 20°C and then filter, the temperature of the filtrate is controlled at 0°C. Measure 7g of hydrobromic acid (converted to 42.3% actual content), add 50ml of ethyl acetate, and mix well. Add the prepared hydrobromic acid solution dropwise to the free alkali solution at a constant speed, control the temperature at 0°C, and continue stirring at 0°C for 8 hours after the addition. Filter to obtain a filter cake 1, which was rinsed three times with ethyl acetate (10 ml each time), then stirred and washed in 50 ml of ethyl acetate at 0° C. for 2 hours. Filtrate again to obtain filter cake 2, which was rinsed three times with a mixed solution of methyl tert-butyl ether / ethyl acetate (v / v=9 / 1) precooled at 0° C., 10 ml each time. The filter cake was stirred in 200ml of methyl tert-butyl ether at 10°C for 15 hours. Then,...
Embodiment 2
[0041] Embodiment 2 Preparation of vortioxetine hydrobromide crystal
[0042] Dissolve 10 g of vortioxetine free base in 360 ml of ethyl acetate, stir to dissolve at 25°C and filter, and control the temperature of the filtrate at 5°C. Measure 6.4g of hydrogen bromide (converted to 42.3% actual content), add 40ml of ethyl acetate, and mix well. Add the prepared hydrobromic acid solution dropwise to the free alkali solution at a constant speed, control the temperature at 5°C, and continue stirring at 5°C for 4 hours after the addition. Filter to obtain filter cake 1, which was rinsed three times with ethyl acetate (15 ml each time), and then stirred and washed in 60 ml of ethyl acetate at 5°C for 1 hour. Filtrate again to obtain filter cake 2, and filter cake 2 is rinsed 3 times with a mixed solution of methyl tert-butyl ether / ethyl acetate (v / v=10 / 1) that has been pre-cooled at 5°C, each 15ml . The filter cake was stirred in 300 ml of methyl tert-butyl ether at 20° C. for 19...
Embodiment 3
[0043] Embodiment 3 Preparation of vortioxetine hydrobromide crystal
[0044] Dissolve 10 g of vortioxetine free base in 380 ml of ethyl acetate, stir to dissolve at 30°C and then filter, the temperature of the filtrate is controlled at 10°C. Measure 7.7g of hydrobromic acid (converted to 42.3% actual content), add 60ml of ethyl acetate, and mix well. Add the prepared hydrobromic acid solution dropwise to the free alkali solution at a constant speed, control the temperature at 10°C, and continue stirring at 10°C for 2 hours after the addition. Filter to obtain filter cake 1, which was rinsed three times with ethyl acetate (20 ml each time), and then stirred and washed in 45 ml of ethyl acetate at 10° C. for 0.5 hours. Filtrate again to obtain filter cake 2, which was rinsed three times with a mixed solution of methyl tert-butyl ether / ethyl acetate (v / v=12 / 1) precooled at 5° C., 20 ml each time. The filter cake was stirred in 350 ml of methyl tert-butyl ether (MTBE) at 30° C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com