Pyrazolyl steroid derivatives and preparation method and application thereof
A technology for pyrazolyl steroids and derivatives, which is applied in the field of drug synthesis and can solve problems such as inability to meet the treatment of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
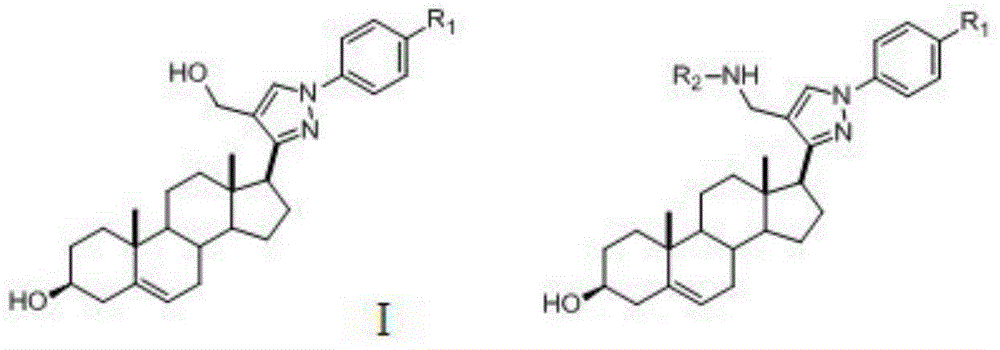
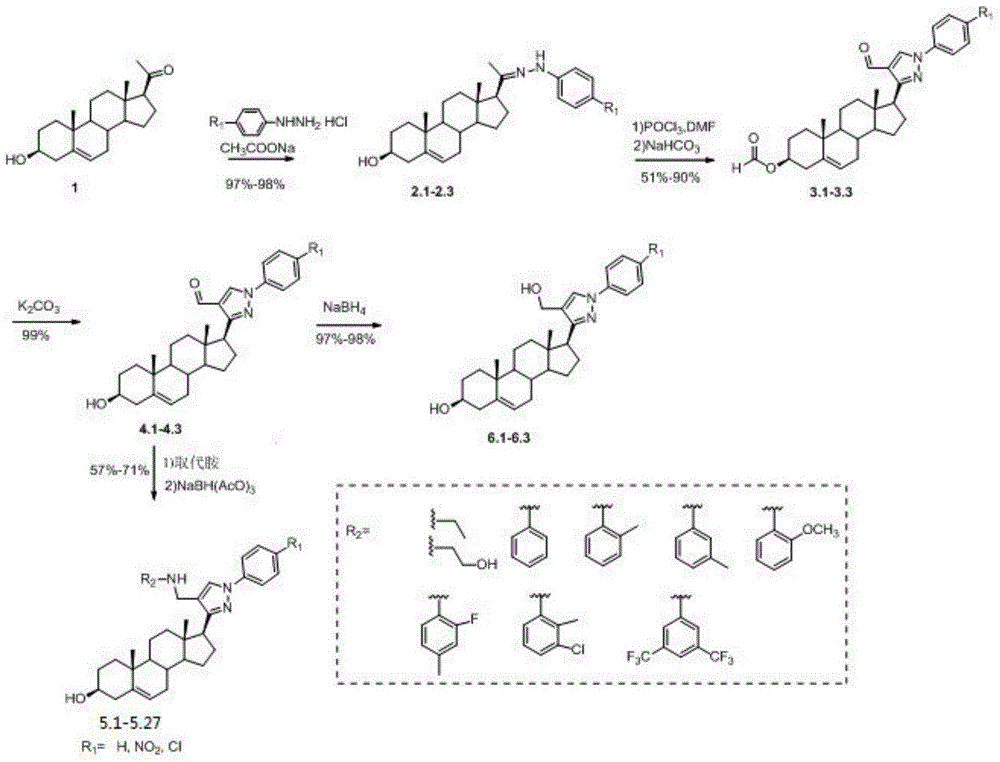
[0060] The preparation of pyrazolyl steroid derivatives of formula (I) by chemical synthesis can be carried out with reference to the following steps.
[0061] (1) Dissolve 6.32g (20mmol) of pregnenolone 1 in a 250mL eggplant-shaped flask with 150mL glacial acetic acid, stir until completely dissolved; then add phenylhydrazine hydrochloride derivatives (22mmol) in batches, stir and sonicate until Dissolve; measure 3.46mL (25mmol) triethylamine, slowly drop it into the reaction system at room temperature, drop it after half an hour, stir at room temperature for 6h until solid precipitates, filter with suction, wash with glacial acetic acid, and dry to obtain the target compound 2.1-2.3 is about 7.8g, the product of this step can be directly used in the next reaction without separation.
[0062] (2) First prepare the visemier reagent: take a well-dried round-bottomed flask, add 20 mL of dried DMF solution, slowly add phosphorus oxychloride solution (4.65 mL, 50 mmol) dropwise un...
Embodiment 2
[0068] The target compound synthesized in Example 1 and the important intermediate are carried out structural identification, and the structure of each compound is passed 1 H-NMR, 13 C-NMR, DEPT135 0 confirm.
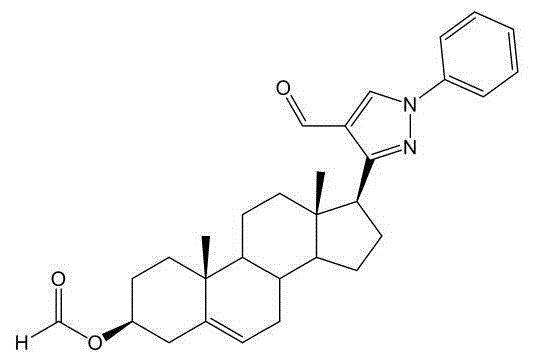
[0069] Compound 3.1
[0070] Compound 3.1 is a key intermediate, and its structure was confirmed by single crystal diffraction. The results are as follows.
[0071]
[0072] 1 H-NMR (CDCl 3 ,500MHz): δ(ppm)9.96(s,1H),8.39(s,1H),8.04(s,1H),7.72(d,2H,J=7.5Hz),7.48(t,2H,J=7.5 Hz), 7.34(t, 1H, J=7.5Hz), 5.43-5.42(m, 1H), 4.75(m, 1H), 3.28(t, 1H, J=10.0Hz), 2.60-2.53(m, 1H ),2.38-2.37(m,2H),2.10-2.01(m,2H),1.92-1.79(m,3H),1.68-1.52(m,6H),1.45-1.14(m,5H),1.03(s ,3H), 0.64(s,3H).
[0073] 13 C-NMR (CDCl 3 ,125MHz): δ(ppm)184.90(CH),160.64(CH),155.53(C),139.42(C),139.33(C),130.65(CH),129.59(CH),129.59(CH),127.49( CH), 123.93(C), 122.82(CH), 119.58(CH), 119.58(CH), 73.92(CH), 56.52(CH), 50.14(CH), 48.38(CH), 44.65(C), 38.07( CH 2 ),37.99(CH 2 ),36.95(CH 2 ), 36...
Embodiment 3
[0215] The anti-tumor cell proliferation activity of formula (I) pyrazolyl steroid derivatives, draws by preliminary test, its cancer cell toxicity IC 50 None of the values exceeded 6uM. In this example, several compounds were selected and evaluated for their biological activity.
[0216] The present embodiment adopts RSB method to measure pyrazolyl steroid derivatives of the present invention formula (I) to A549 (human lung adenocarcinoma cell), Hela (cervix cancer cell line), HepG2 (human liver cancer cell) three kinds of tumor cells growth inhibitory activity.
[0217] specific method:
[0218] Accurately weigh 1-3mg of the compound to be tested, use DMSO as solvent, prepare a solution with a concentration of 10mMol / L, and let it stand at room temperature for half an hour until the sample is completely dissolved, then store it for later use.
[0219] Take A549, Hela, and HepG2 cells in the logarithmic growth phase, wash and digest with trypsin to make a cell suspension...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com