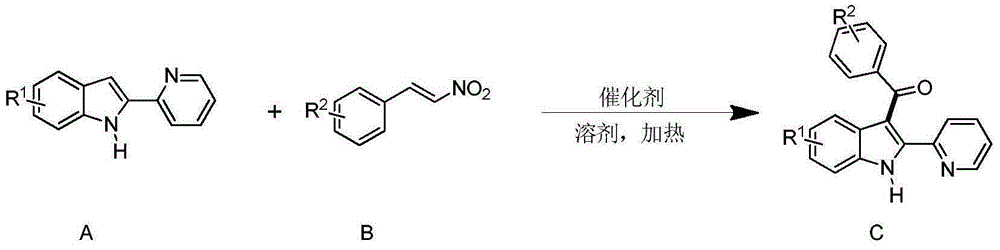
Synthesizing method for 3-aroyl indole compound
An aroyl indole compound, green synthesis technology, applied in the direction of organic chemistry, etc., can solve the problem of limited use and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
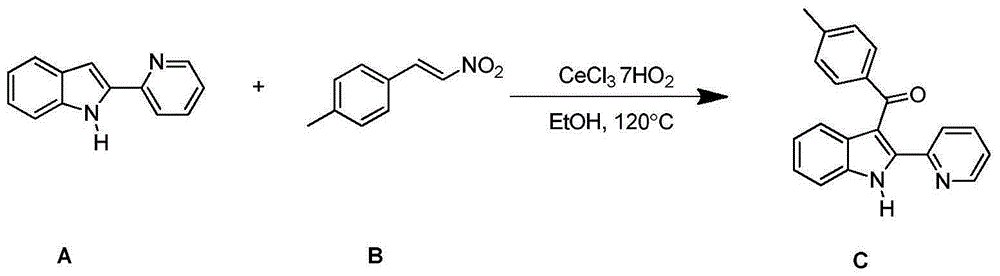
Embodiment 1
[0022] 4-methyl-β-nitrostyrene and 2-(2-pyridyl)-1H-indole, solvent ethanol
[0023]
[0024] Add CeCl to the thick-walled pressure-resistant reaction tube in sequence 3 ·7H 2 O (7.5mg, 0.02mmol), reactant A (38.8mg, 0.2mmol), reactant B (48.9mg, 0.3mmol); then add solvent ethanol 2ml, put it in 120°C oil bath for 12h. After the reaction was complete as detected by TLC, it was separated and purified by column chromatography, the developer was petroleum ether: ethyl acetate = 4:1, and the separation yield was 74.75%. 1 HNMR (400MHz, CDCl 3 ):δ11.06(s,1H),8.57(d,J=4.2Hz,1H),7.82-7.78(m,3H),7.51(td,J 1 =7.7Hz,J 2 =1.7Hz,1H),7.44(d,J=8.0Hz,1H),7.38(d,J=8.1Hz,1H),7.24-7.14(m,4H),7.10-7.06(m,1H),2.38 (s,3H); 13 CNMR (100MHz, CDCl 3 ): δ193.5, 149.1, 148.7, 143.4, 138.8, 137.0, 136.6, 135.1, 130.2, 129.0, 128.9, 124.5, 123.8, 123.1, 121.6, 121.4, 114.5, 111.5, 21.6.
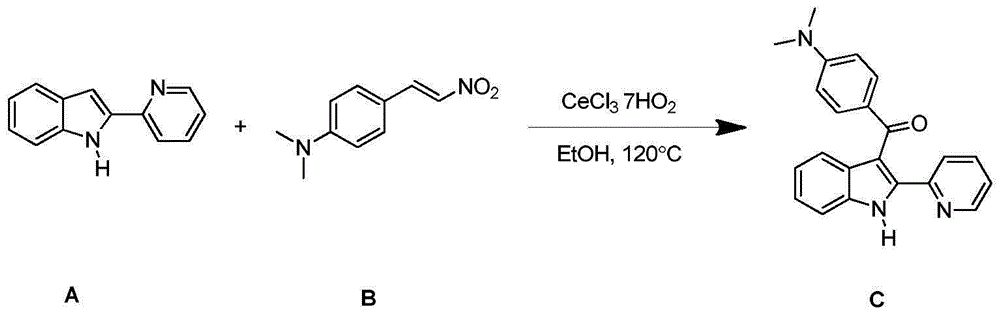
Embodiment 2
[0026] (E)-N,N-Dimethyl-4-(2-nitrovinyl)aniline and 2-(2-pyridyl)-1H-indole, solvent ethanol
[0027]
[0028] Add CeCl to the thick-walled pressure-resistant reaction tube in sequence 3 ·7H 2 O (7.5mg, 0.02mmol), reactant A (38.8mg, 0.2mmol)), reactant B (57.6mg, 0.3mmol); then add solvent ethanol 2ml, put it in 120℃ oil bath for 12h. After the reaction was complete as detected by TLC, it was separated and purified by column chromatography. The developing solvent was petroleum ether: ethyl acetate = 4:1, and the separation yield was 71.72%. 1 HNMR (400MHz, CDCl 3 ):δ0.63(s,1H),8.57(d,J=4.1Hz,1H),7.87(d,J=8.6Hz,3H),7.56-7.37(m,3H),7.24-7.12(m, 2H), 7.07-7.03(m, 1H), 6.59(d, J=8.8Hz, 2H), 3.02(s, 3H); 13 CNMR (100MHz, CDCl 3 ): δ192.2, 153.4, 149.3, 148.7, 136.8, 136.6, 134.9, 132.6, 129.0, 126.7, 123.7, 123.5, 122.7, 121.5, 120.8, 115.3, 111.3, 110.5, 40.0.
Embodiment 3
[0030] 4-Chloro-β-nitrostyrene and 2-(2-pyridyl)-1H-indole, solvent ethanol
[0031]
[0032] Add CeCl to the thick-walled pressure-resistant reaction tube in sequence 3 ·7H 2 O (7.5mg, 0.02mmol), reactant A (38.8mg, 0.2mmol)), reactant B (57.7mg, 0.3mmol); then add solvent ethanol 2ml, put it in 120℃ oil bath for 12h. After the reaction was complete as detected by TLC, it was separated and purified by column chromatography. The developing solvent was petroleum ether: ethyl acetate = 4:1, and the separation yield was 82.21%. 1 HNMR (400MHz, CDCl 3 ):δ10.4(s,1H),8.6(d,J=3.2Hz),1H,7.85-7.80(m,3H),7.59-7.57(t,J=7.4Hz,1H),7.47-7.40( m,2H),7.36-7.35(m,J=8.20Hz,2H),7.29-7.21(m,2H),7.14-7.10(m,1H); 13 CNMR (100MHz, CDCl 3): δ192.2, 149.0, 148.7, 139.2, 138.9, 138.1, 136.8, 134.9, 131.4, 128.8, 128.6, 124.5, 124.1, 123.4, 121.8, 121.5, 113.8, 111.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com