High-sensitivity measurement method of gad antibody as an early diagnosis index of type 1 diabetes
An antibody and autoantibody technology, applied in the field of high-sensitivity determination of GAD antibodies, can solve problems such as difficulty in finding or detecting autoantibodies and low detection sensitivity, and achieve the effect of improving diagnostic accuracy, high sensitivity, and avoiding ignoring symptoms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
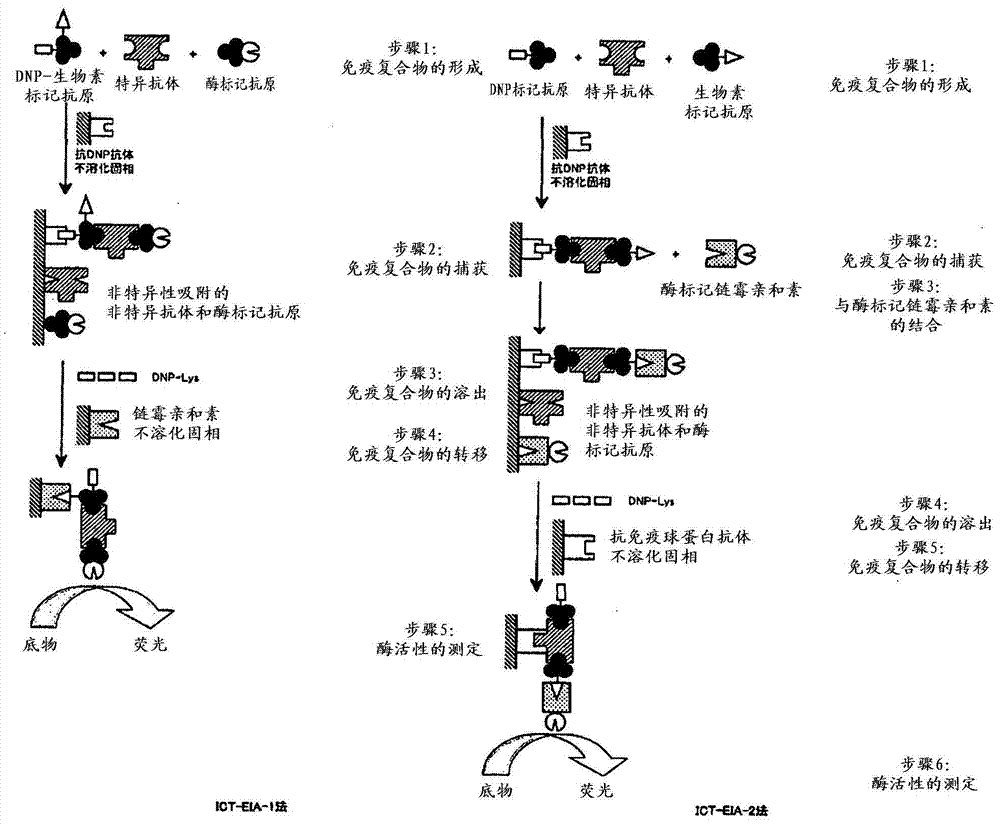
[0065] The "immune complex transfer enzyme immunoassay method (ICT-EIA method)" of the present invention refers to a method related to a highly sensitive improved method of a non-competitive binding method (sandwich) enzyme immunoassay (EIA) (refer to non-competitive Patent Document 1). like figure 1 As shown, the non-specific adsorption (background) of the antibody used can be reduced by the ICT-EIA method, so the measurement of the amol level or less (zmol) of various polymer physiologically active substances becomes possible (see Hashida S, et al., Biotechnology Annual Review Volume 1, (1995) pp403-451, Elsevier Science Publishers B.V., Amsterdam).
[0066] The "GAD antibody" of the present invention refers to an autoantibody against glutamic acid decarboxylase (glutamic acid decarboxylase: GAD). GAD is an enzyme that synthesizes γ-aminobutyric acid (GABA) from L-glutamic acid, and is mostly expressed in brain or pancreatic islet β cells. The role of GABA in pancreatic β...
Embodiment 1
[0073] [Example 1: ICT-EIA method for GAD antibody]
[0074] 【(1) Reagents】
[0075] (a) General reagents:
[0076] Bovine serum albumin (BSA) was purchased from Nacalai tesque (Kyoto), and streptavidin was purchased from Wako Pure Chemical Industries (Osaka). Other general reagents were purchased from Nacalai tesque and Wako Pure Chemical Industries.
[0077] (b) Antibodies:
[0078] Rabbit anti-2,4-dinitrophenyl (DNP)-bovine serum albumin (BSA) serum was purchased from Goat Company (Gunma).
[0079] (b) Antigen:
[0080] Recombinant-human-GAD65 was purchased from RSR Limited (Cardiff, UK).
[0081] (c) Buffer:
[0082] With 0.1M NaCl, 0.1% BSA, 1mM MgCl 2 and 0.1% NaN3 0.01M sodium phosphate buffer (pH7.0) as buffer A, containing 0.4M NaCl, 0.1% BSA, 1mM MgCl 2 and 0.1% NaN3 0.01M sodium phosphate buffer (pH 7.0) as buffer B, containing 0.1M NaCl, 0.01% BSA, 1mM MgCl 2 and 0.1% NaN3 0.01M sodium phosphate buffer (pH 7.0) as buffer C, 0.01M sodium phosphate buffer (p...
Embodiment 2
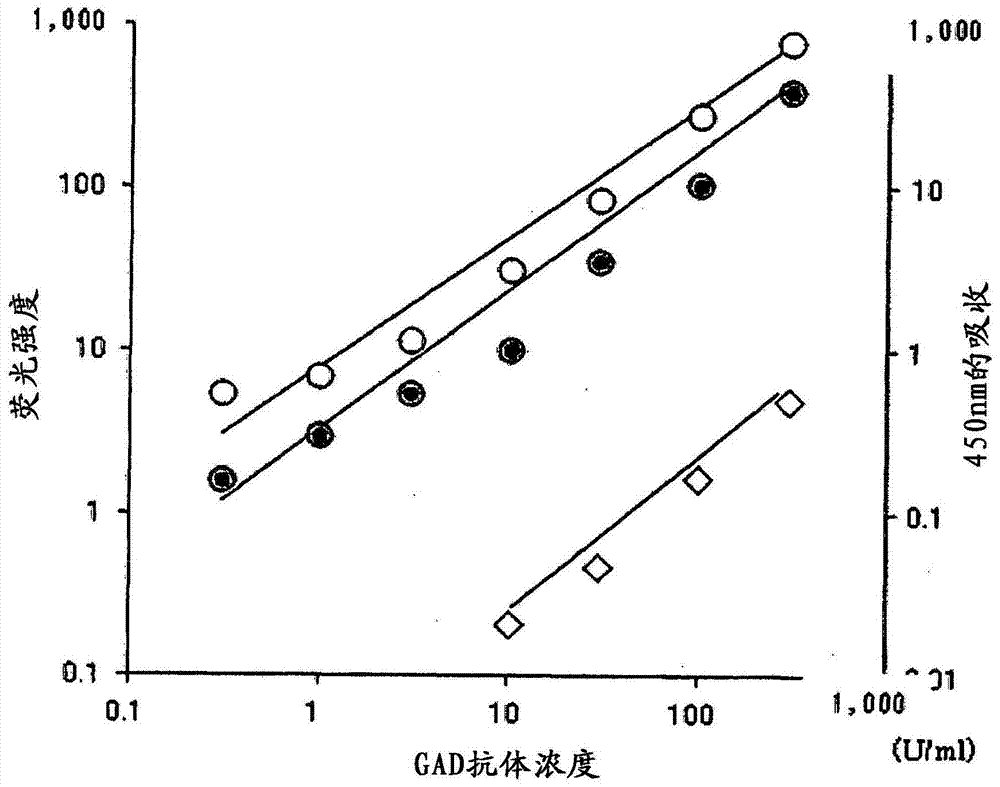
[0120] [Example 2: Comparison of the detection sensitivity of ICT-EIA-1 method, ICT-EIA-2 method and ELISA method]
[0121] 【(1) Subjects, etc.】
[0122] (a) Target person:
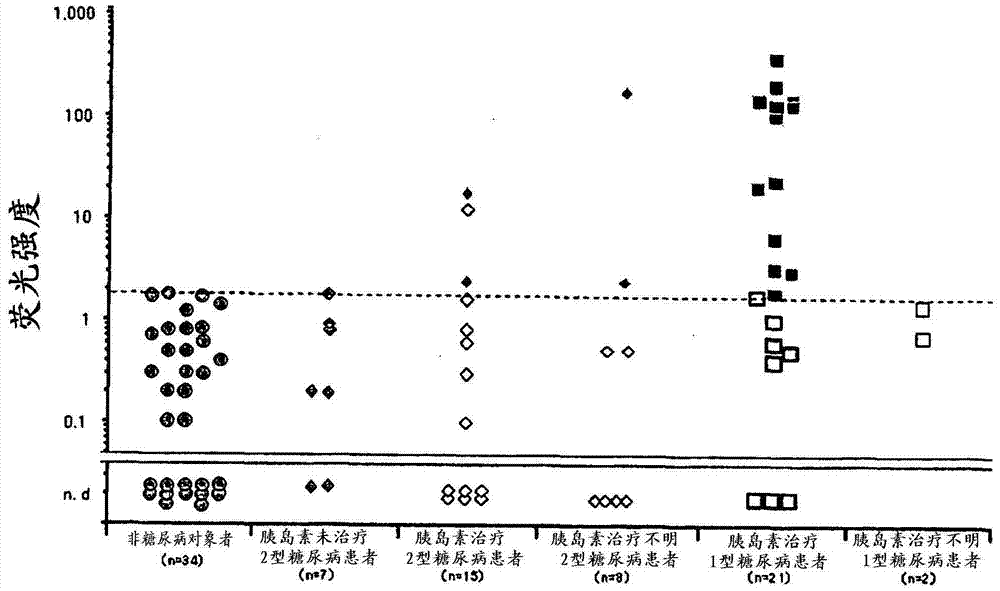
[0123] From non-diabetic subjects (73 patients), hyperinsulinemia patients (9 patients), Graves' disease patients (30 patients) under administration of antithyroid agent (methimazole), Hashimoto's disease patients (20 patients), 2 Type 2 diabetes patients (30; insulin not treated: 7, insulin therapy: 15, insulin therapy unknown: 8), type 1 diabetes patients (24; insulin therapy: 21, insulin therapy unknown: 3 ) to collect samples.
[0124] (b) Informed Consent:
[0125] The experiment conducted in this study was performed with the approval of the ethics committee of Tokushima Bunri University (approval number No. 4). Informed consent was obtained among the test subjects, and the experiment was carried out after obtaining the consent.
[0126] (c) Statistical processing:
[0127] The cutoff value was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com