Novel recombinant double-function fusion protein, and preparation method and application thereof
A technology of fusion protein and protein, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
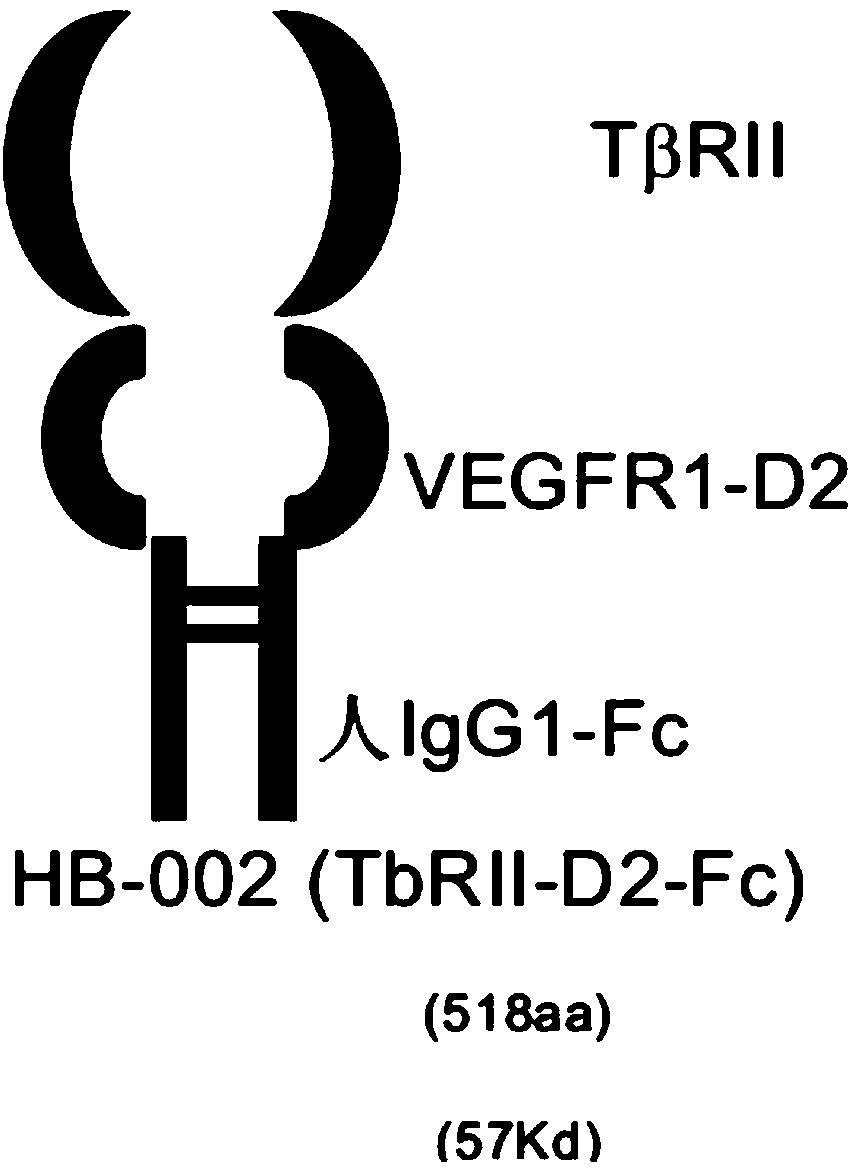
[0166] Construction of TβRII-D2-Fc expression vector
[0167] The coding sequence of the TβRII extramembrane region gene consists of 483 nucleotides, as shown in positions 70-552 in SEQ ID NO.:2. The VEGFR1-D2 gene coding sequence consists of 300 nucleotides, as shown in positions 553-852 in SEQ ID NO.:2, including 279 nucleotides of the D2 coding sequence, 15 nucleotides of the upstream flanking sequence, The downstream flanking sequence is 6 nucleotides. 69 signal peptide coding sequences from TβRII (ie, positions 1-69 in SEQ ID NO.: 2) are added to the 5' end of the TβRII outer region, forming 852 nucleotides.
[0168] A "HindIII" gene cloning site and an "EcoRI" gene cloning site were added to the amino terminus of the 852 nucleotides to form a gene fragment containing 873 nucleotides.
[0169] The synthetic product (synthesized by Nanjing GenScript Biotechnology Co., Ltd.) was digested with HindIII / EcoRI and cloned into the pHB-Fc plasmid vector to form the pHB-TbRII-D2...
Embodiment 2
[0174] Expression of TβRII-D2-Fc, D2-TβRII-Fc, TβRII-Fc-D2
[0175] The host cells used for protein expression were CHO-K1 cells (Cat#CCL-61) purchased from ATCC Company. After a series of domestication steps, the cells are domesticated into CHO-K1 cells that can be cultured in suspension in serum-free medium (EX-CELLTM302).
[0176] Using the cells, the plasmids pHB-TβRII-D2-Fc, pHB-D2-TβRII-Fc, and pHB-TβRII-Fc-D2 were respectively transferred into the cells by electroporation. The specific method is: collect the cells in the logarithmic growth phase under sterile conditions, resuspend in the complete medium after centrifugation (1200rpm x5min), and adjust the cell density to 1×10 7 cells / ml. Transfer 350ul of the cell suspension to a 0.4cm electroporation cup, and pulse once under the set electroporation conditions (voltage range 200 to 350V, generally 260V, time about 20ms). Add 10–30ug of plasmid DNA to the electroporation cup containing the cells, mix gently, put the ...
Embodiment 3
[0181] TβRII-D2-Fc and target (VEGF and TGF-β1) binding activity detection
[0182] Enzyme-linked immunosorbent assay (ELISA) method was used to determine the binding properties of the fusion protein and the target (VEGF and TGF-β1). Specific steps are as follows:
[0183] TGF-β1 (Cat: 10804-HNAH, Sino biological Inc.) and VEGF-165 (Cat: 11066-HNAH, Sino biological Inc.) were coated with coating buffer CBS (Sigma-Aldrich Co., Product code: 1001329288C3041-100CAP). .) were diluted to 500ng / ml respectively, and 100ul was added to the ELISA plate (Nunc TM , Cat:442404), 50ng per well. Place the coated plates in a 4°C refrigerator overnight. When detecting, wash the coated plate once with 0.05% PBS-T, and then block it with 3% skimmed milk at room temperature for 1 hour. Add 2-fold serially diluted TbRII-D2-Fc protein (100 nM, 50 nM, 25 nM, up to 0.0244 nM) into the coated plate, 100 ul per well. After incubating at room temperature for one hour, the sample was discarded, was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com