Application of two quadruple lactone antibiotics as anti-MRSA drugs, and extraction and separation method of two quadruple lactone antibiotics
A technology of antibiotics and esters, used in antibacterial drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc., to achieve the effect of high extraction and separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of two kinds of quadruple lactone compounds
[0046] 1. Source and identification of the strain
[0047] The compounds of formula 1 and formula 2 were both isolated from the fermentation product of a Dracaena genus genus endophyte, which was provided by Zhang Xiaomei’s research group at the School of Basic Medicine, Yunnan University of Traditional Chinese Medicine, and compared by 16S rRNA sequencing analysis , identified as Streptomyces puniceus S009.
[0048] Isolation and Identification of Streptomyces puniceus S009
[0049] 1.1. Strain isolation
[0050] Healthy plant samples were rinsed with running water, then air-dried at room temperature for 48 hours, and then cleaned ultrasonically at 160W for 5 minutes to remove soil and organic residues on the plant surface. And carry out the surface disinfection of plant samples according to the following process: soak in 70% ethanol for 5 minutes, wash the sample three times in sterile wate...
Embodiment 2
[0065] Embodiment 2: Structural identification of two kinds of quadruple lactone compounds
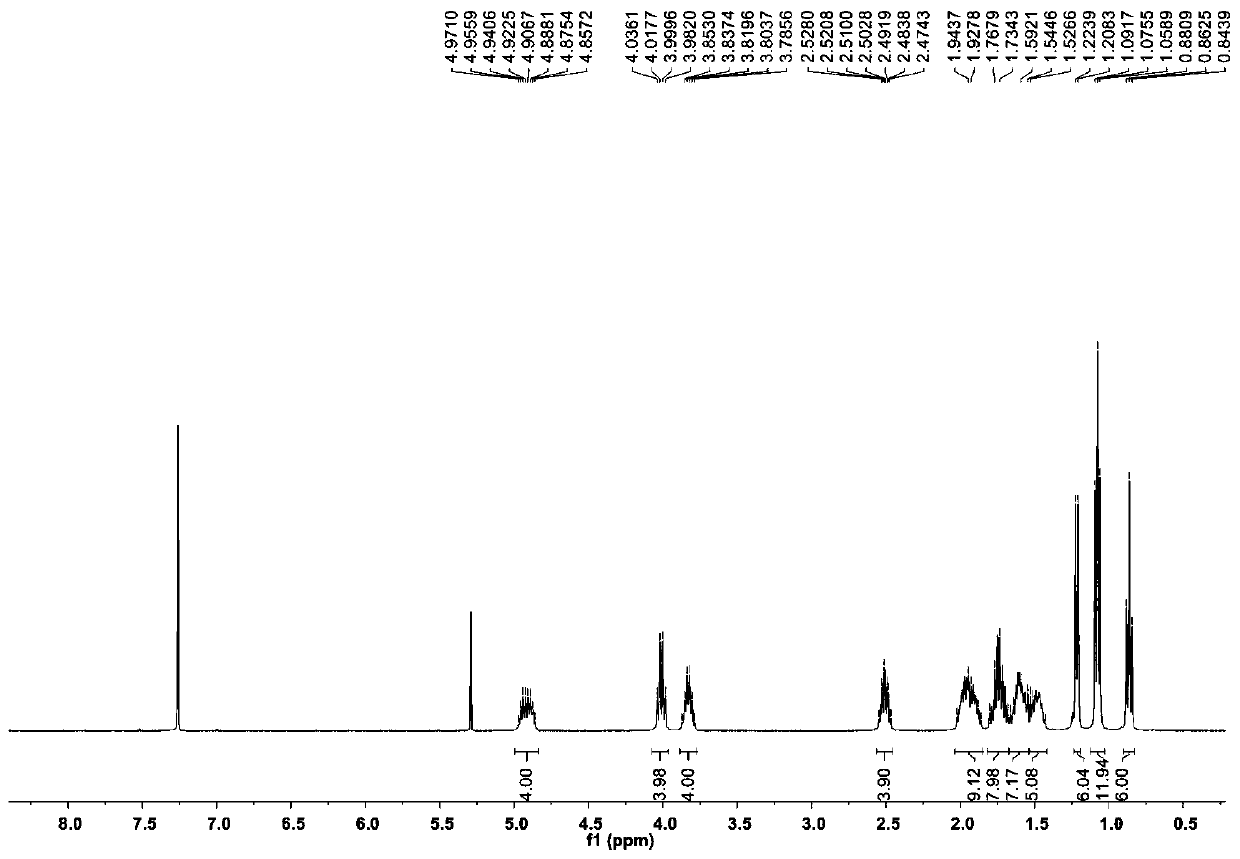
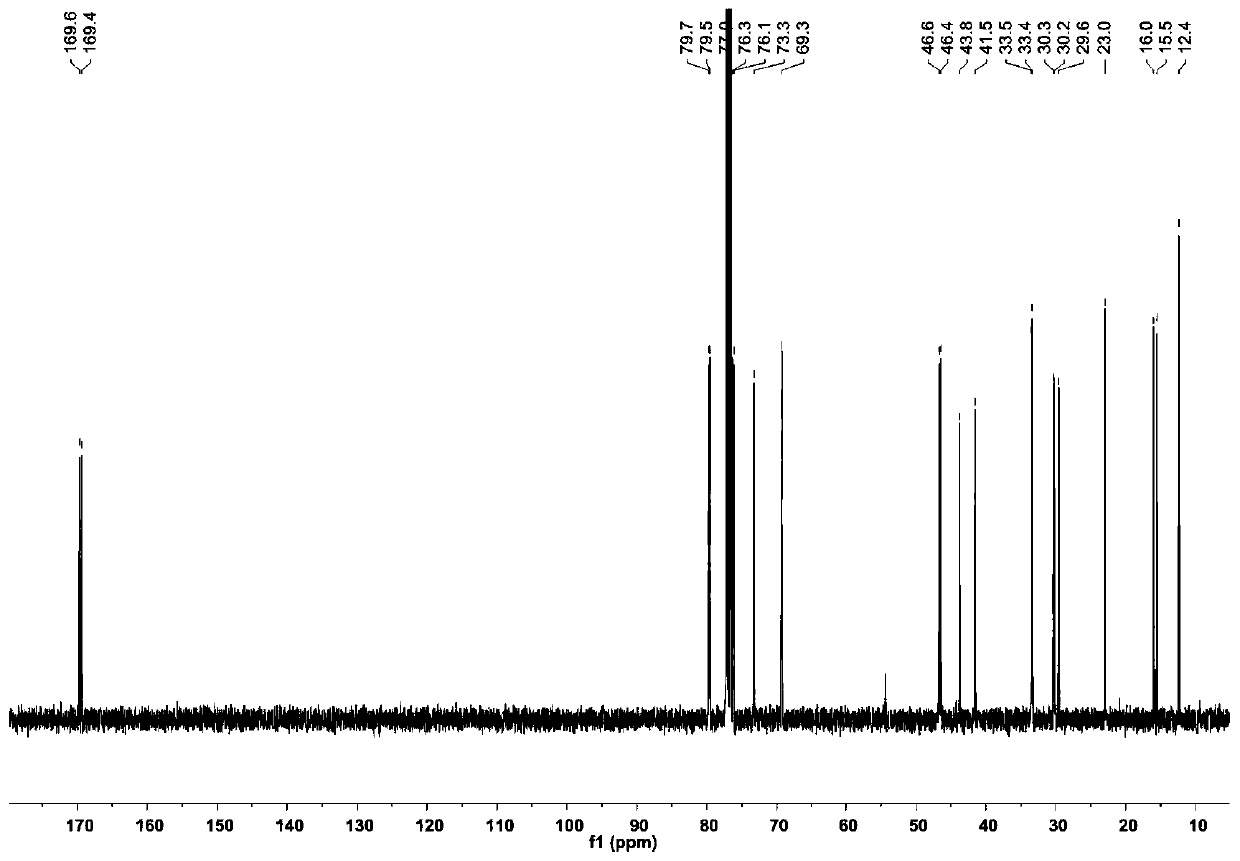
[0066] The compound Dinactin (1) prepared in Example 1 is a colorless oily substance, which is easily soluble in chloroform, acetone and DMSO. Figure 2 to Figure 7 is the structural analysis spectrum of compound Dinactin (1), HR-ESI-MS: m / z 787.4590[M+Na] + (Positive ion mode), determine its molecular formula as C 42 h 68 o 12 . From 1 H NMR and 13 The C NMR data shows that the NMR data of compound 1 is basically consistent with the data of dinactin (diactin) reported in the literature, and the NOESY related signal And the optical rotation data further confirmed that the three-dimensional configuration of compound 1 was consistent with that reported in the literature. Therefore, identification compound 1 is dinactin, as shown in Table 1:
[0067] Table 1. Compounds of Dinactin 1 H(400MHz) and 13 C (100MHz) NMR data (solvent: CDCl 3 )
[0068]
[0069]
[0070] The...
Embodiment 3
[0072] Embodiment 3: the research of anti-MRSA effect of compound Dinactin (1) and Trinactin (2) in vitro
[0073] 1. Experimental indicator bacteria
[0074] 9 strains of MRSA drug-resistant bacteria used in the present invention: 1505, 1450, 1591, 1957, 2024, I-20, I-67, 28299, 28300, are all isolated from patients in the First People's Hospital of Qujing City. resistant to vancomycin.
[0075] 2. Samples to be tested
[0076] Dinactin(1), Trinactin(2), Vancomycin (positive control), DMSO (negative control)
[0077] 3. Medium
[0078] Liquid LB medium: peptone 10g; yeast extract 5g; NaCl 10g; agar 15g; water 1000mL; PH7.2-7.6.
[0079] 4. Experimental method
[0080] 1) Preparation of bacterial solution
[0081] Inoculate the purified indicator bacteria into the corresponding liquid medium: MRSA was inoculated in the LB medium, cultured at 37°C for 12-24 hours, then diluted with the corresponding liquid medium: Bacteria 0.5×10 6 CFU / mL.
[0082] 2) Preparation of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com