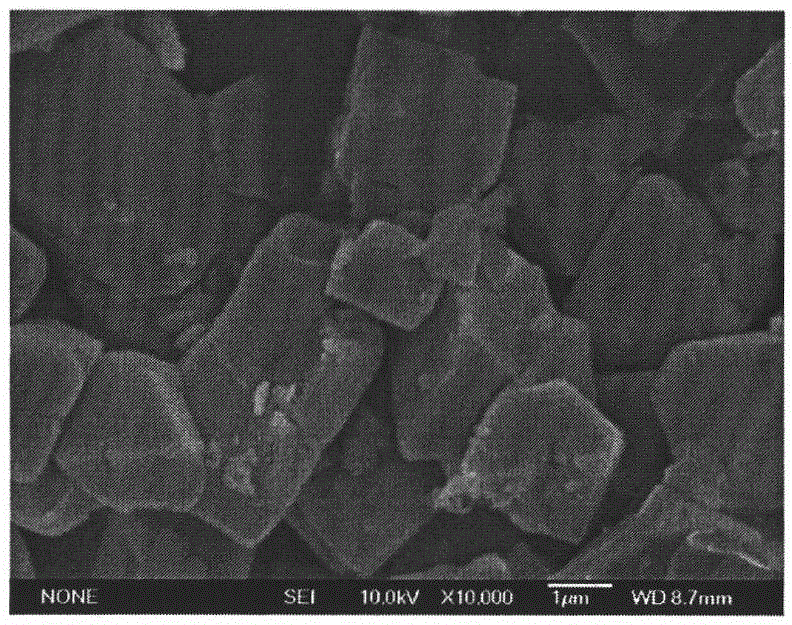
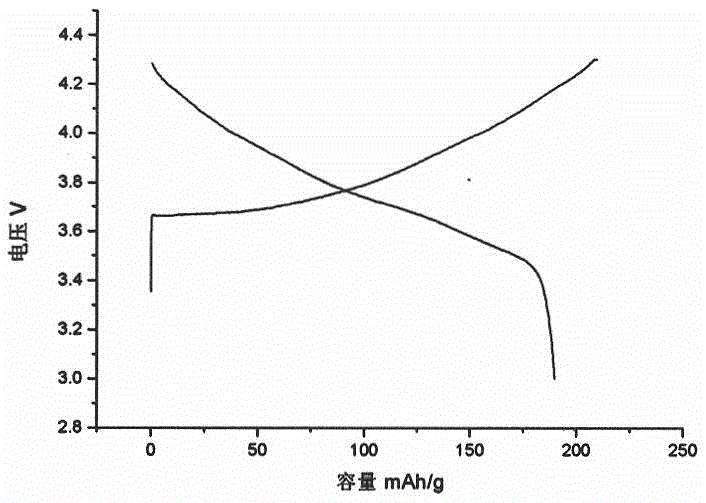
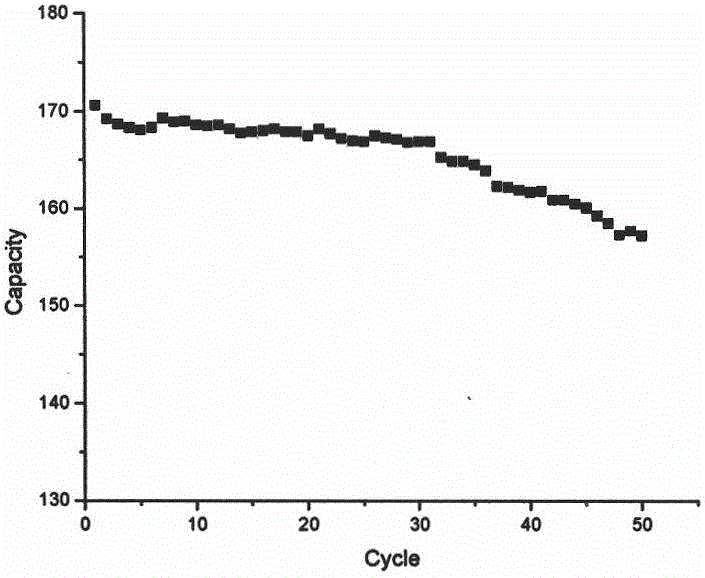
Preparation method of primary large-particle lithium nickel cobalt aluminate anode material
A technology of nickel-cobalt-lithium aluminate and nickel-cobalt-aluminum, which is applied in the field of positive electrode materials for high specific capacity lithium-ion batteries and its preparation, can solve the problems of large capacity loss of NCA materials and affect NCA materials, and achieve a simple and easy process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Dissolve nickel salt, cobalt salt, and aluminum salt in deionized water and mix evenly to obtain a 2M nickel-cobalt-aluminum salt solution, wherein the molar ratio of nickel-cobalt-aluminum ions is: 8:1.5:0.5, and will contain 4M The 10M sodium hydroxide solution of ammonia water is used as the alkali solution and the nickel-cobalt-aluminum solution to flow into the high-speed stirring reactor, adjust the flow rate of the alkali solution to control the pH value in the reactor between 10-11, and stop after the injection of the nickel-cobalt-aluminum solution is completed. Injecting liquid, separating the obtained slurry into solid and liquid, washing the obtained solid material and drying in an oven at 120°C for 8 hours to obtain a nickel-cobalt-aluminum precursor. Mix the nickel-cobalt-aluminum precursor and the lithium source at a molar ratio of 1:1.05, put them into a roaster, roast them at 300°C for 8 hours in an air atmosphere, and then lower them to room ...
Embodiment 2
[0020] Dissolve nickel salt, cobalt salt, and aluminum salt in deionized water and mix evenly to obtain a 2M nickel-cobalt-aluminum salt solution, wherein the molar ratio of nickel-cobalt-aluminum ions is: 8:1:1. Sodium hydroxide solution is used as alkaline solution and nickel-cobalt-aluminum solution and flow into the high-speed stirring reactor, adjust the flow rate of alkaline solution to control the pH value in the reactor between 11-12, stop the liquid injection after the nickel-cobalt-aluminum solution is injected, and The obtained slurry is subjected to solid-liquid separation, and the obtained solid material is washed and dried in an oven at 150 degrees for 8 hours to obtain a nickel-cobalt-aluminum precursor. Mix the nickel-cobalt-aluminum precursor and the lithium source in a molar ratio of 1:1.1, put them into a roaster, roast them at 500°C for 20 hours in an air atmosphere, then lower them to room temperature, and pass the roasted materials through a 500-mesh sieve...
Embodiment 3
[0023] Dissolve nickel salt, cobalt salt, and aluminum salt in deionized water and mix evenly to obtain a 2M nickel-cobalt-aluminum salt solution, wherein the molar ratio of nickel-cobalt-aluminum ions is: 7:2:1. Sodium hydroxide solution is used as alkali solution and nickel-cobalt-aluminum solution and flow into the high-speed stirring reactor, adjust the flow rate of alkali solution to control the pH value in the reactor between 12.5-13, stop the liquid injection after the nickel-cobalt-aluminum solution is injected, and The obtained slurry is subjected to solid-liquid separation, and the obtained solid material is washed and dried in an oven at 150 degrees for 12 hours to obtain a nickel-cobalt-aluminum precursor. Mix the nickel-cobalt-aluminum precursor and the lithium source at a molar ratio of 1:1, put them into a roaster, and roast them at 400°C for 10 hours in an air atmosphere, then lower them to room temperature, and pass the roasted materials through a 500-mesh siev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com