Preparation method of biodegradable polyether urethane sponge
A polyether-type polyurethane biodegradable technology, applied in drug delivery, pharmaceutical formulation, application, etc., can solve the problems of difficult control of the content of hydrophilic segments and difficult adjustment of soft and hard segments, so that the added amount is easier to control and easy Effects of operation and improvement of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
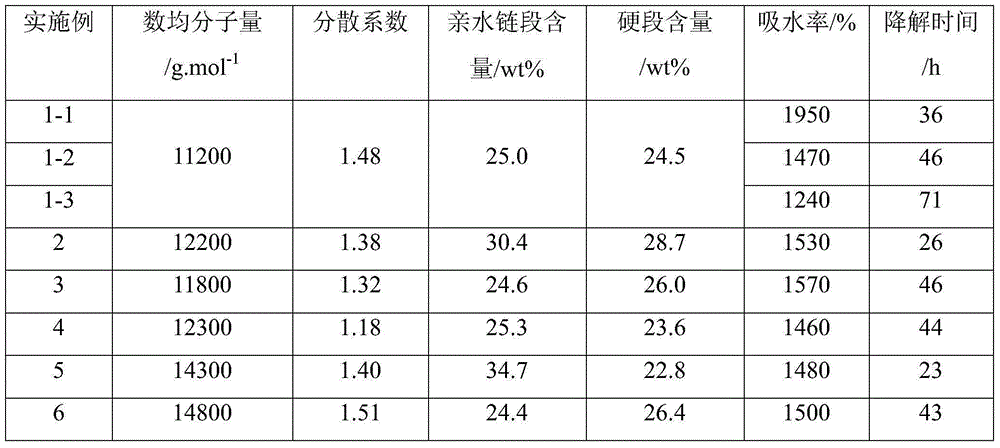
Examples
Embodiment 1
[0063] 1) Synthesis of double-terminated hydroxyl prepolymer
[0064] Put 0.45g (0.005mol) of 1,4-butanediol in a vacuum reaction flask, stir with a magnet, remove water under vacuum (20Pa) at 100°C for 4 hours, cool to room temperature, and balance with nitrogen gas. Add 24 g of D,L-lactide, 24 g of ε-caprolactone, and 90 μL of stannous octoate (0.2% of the mass of the raw material). Vacuumize and equilibrate with dry nitrogen, repeat three times. Vacuumize to 20Pa, seal the vacuum reaction bottle, heat the oil bath to 140°C, and react for 36 hours to obtain a double-terminated hydroxyl prepolymer.
[0065] 2) Preparation of double-terminated isocyanate-based prepolymer
[0066] Balance the double-terminated hydroxyl prepolymer synthesized in step 1) with nitrogen gas, cool down to 80°C, mix well with 24g PEG (molecular weight: 600) (0.04mol) after vacuum dehydration, and then add 60.5g (0.36mol, - NCO is 8 times the total molar amount of -OH) 1,6-hexamethylene diisocyanat...
Embodiment 2
[0072] 1) Synthesis of double-terminated hydroxyl prepolymer
[0073] Put 0.45g (0.005mol) of 1,4-butanediol in a vacuum reaction flask, stir with a magnet, remove water under vacuum (20Pa) at 100°C for 4 hours, cool to room temperature, and balance with nitrogen gas. Add 24 g of D,L-lactide, 24 g of ε-caprolactone, and 90 μL of stannous octoate (0.2% of the mass of the raw material). Vacuumize and equilibrate with dry nitrogen, repeat three times. Vacuum to 20Pa, seal the vacuum reaction bottle, heat the oil bath to 130°C, and react for 24 hours to obtain a double-terminated hydroxyl prepolymer.
[0074] 2) Preparation of double-terminated isocyanate-based prepolymer
[0075] The double-terminated hydroxyl prepolymer synthesized in step 1) was equilibrated with nitrogen gas, cooled to 80°C, mixed evenly with 36g of PEG (molecular weight: 600) (0.06mol) after vacuum dehydration, and then added 87.4g (0.52mol, - NCO is 8 times the total molar amount of -OH) 1,6-hexamethylene...
Embodiment 3
[0081] 1) Synthesis of double-terminated hydroxyl prepolymer
[0082] Put 0.225g (0.0025mol) of 1,4-butanediol in a vacuum reaction flask, stir with a magnet, remove water under vacuum (20Pa) at 100°C for 4 hours, cool to room temperature, and balance with nitrogen gas. Add 24 g of D,L-lactide, 24 g of ε-caprolactone, and 80 μL of stannous octoate (0.15% of the mass of the raw material). Vacuumize and equilibrate with dry nitrogen, repeat three times. Vacuum to 20Pa, seal the vacuum reaction bottle, heat the oil bath to 120°C, and react for 16 hours to obtain a double-terminated hydroxyl prepolymer.
[0083] 2) Preparation of double-terminated isocyanate-based prepolymer
[0084] The double-terminated hydroxyl prepolymer synthesized in step 1) was equilibrated with nitrogen gas, cooled to 80°C, mixed with 24g PEG (molecular weight: 600) (0.04mol) after vacuum dehydration, and then added 46.3g (0.276mol, - NCO is 6.5 times the total molar amount of -OH) 1,6-hexamethylene dii...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com