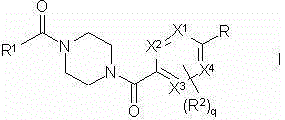
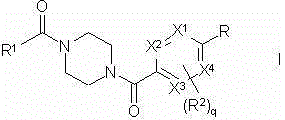
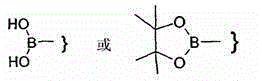
Piperazine derivatives as FASN inhibitors
A compound and mixture technology, applied in anti-inflammatory agents, suppository delivery, drug combination, etc., can solve problems such as weakening HCV replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0206] 1-[4-(4-Isoquinolin-6-yl-benzoyl)-piperazin-1-yl]-propan-1-one ("A1")
[0207]
[0208] 1.1 Dissolve tert-butyl-1-piperazinecarboxylate (0.5 g; 2.69 mmol) in 10 ml of dichloromethane (DCM). N-ethyldiisopropylamine (1.37 ml; 8.06 mmol) for synthesis was added, and the mixture was cooled to 0°C. Now, the 4-bromobenzoyl chloride (0.66 g; 2.95 mmol) used for the synthesis was dissolved in 10 ml DCM and added dropwise to the reaction, followed by stirring overnight at room temperature. On LC-MS, the reaction was complete. The reaction mixture was extracted with 50 mL of water. The organic layer was washed with MgSO 4Drying, filtering off, and then reducing to dryness under vacuum afforded 1,0 g (100%) of tert-butyl 4-(4-bromo-benzoyl)-piperazine-1-carboxylate 1 as a beige solid.
[0209] 1.2 Dissolve tert-butyl 4-(4-bromo-benzoyl)-piperazine-1-carboxylate 1 (0.5 g; 1.35 mmol) in 15 ml HCl / isopropanol (5-6N), and then °C and stirred for 2 hours. The reaction mixture ...
Embodiment A
[0222] Example A: Injection vial
[0223] A solution of 100 g of the active ingredient of formula I and 5 g of disodium hydrogen phosphate in 3 l of double distilled water was adjusted to pH 6.5 using 2 N hydrochloric acid, sterile filtered, transferred into injection vials and lyophilized under sterile conditions , and sealed under sterile conditions. Each injection vial contains 5 mg of active ingredient.
Embodiment B
[0224] Embodiment B: Suppository
[0225] A mixture of 20 g of active ingredient of formula I and 100 g of soy lecithin and 1400 g of cocoa butter is melted, poured into molds and allowed to cool. Each suppository contains 20 mg of active ingredient.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com