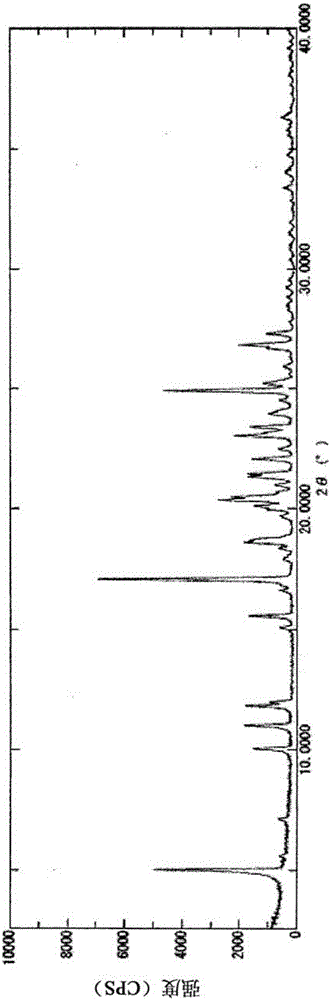
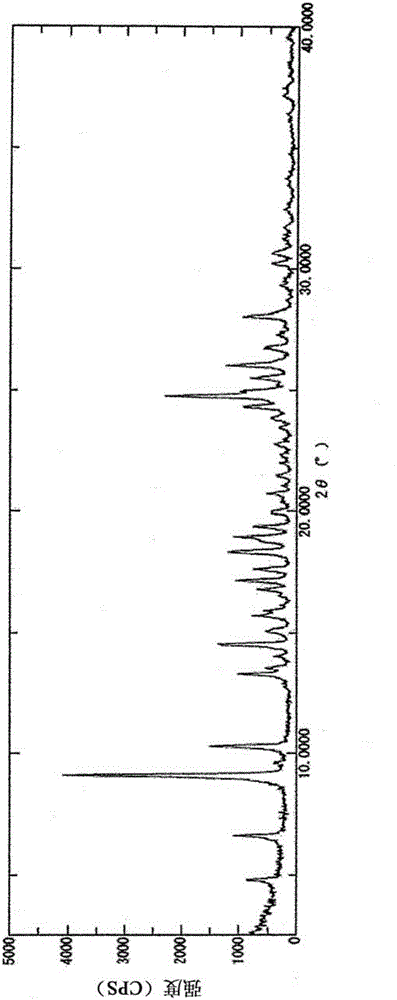
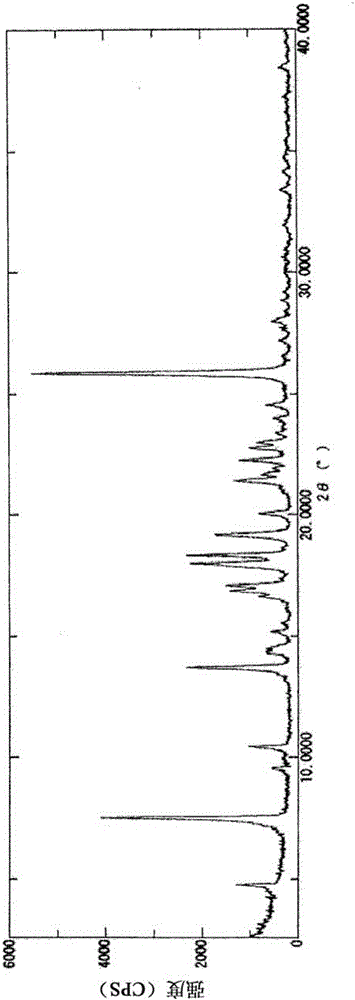
2-acylaminothiazole derivative and salt thereof
An amino and alkyl technology, which is applied in the field of 2-acylaminothiazole derivatives or their salts, can solve the problems such as ineffectiveness of urination disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0329] Hereinafter, based on examples, the production method of the compound of formula (I) will be described in further detail. In addition, this invention is not limited to the compound described in the following Example. In addition, the production method of the raw material compound is as shown in the production example. Furthermore, the production method of the compound of formula (I) is not limited to the production method shown in the specific examples below, and the compound of formula (I) can also be produced by a combination of these production methods or a method obvious to those skilled in the art.
[0330] In this specification, naming software such as ACD / Name (registered trademark, Advanced Chemistry Development, Inc.) may be used for naming compounds.
[0331] In addition, the following abbreviations may be used in Examples, Production Examples, and Tables described later.
[0332] PEx: production example number, Ex: example number, PSyn: production example n...
manufacture example 1
[0339] To a solution of 1-[4-hydroxy-3-(trifluoromethyl)phenyl]ethanone (1 g) in acetonitrile (10 mL) was added 1-bromopropane (0.90 mL), potassium carbonate (1.7 g) and tetrabutyl ammonium iodide (180 mg), stirred overnight at room temperature. The insoluble matter was filtered off, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain 1-[4-propoxy-3-(trifluoromethyl)phenyl]ethanone (1.16 g) as an oily substance.
manufacture example 2
[0341] 1-[4-Hydroxy-3-(trifluoromethyl)phenyl]ethanone (1 g), ethyl iodide (1.19 mL), cerium carbonate (1.92 g) and N,N-dimethylformamide ( 15 mL) was stirred at 60°C for 3 hours. The reaction mixture was cooled to room temperature, water was added, and extracted with ethyl acetate. The organic layer was washed with water and a saturated aqueous sodium chloride solution, and dried over anhydrous sodium sulfate, the insoluble matter was filtered off, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain 1-[4-ethoxy-3-(trifluoromethyl)phenyl]ethanone (1.1 g) as a solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com