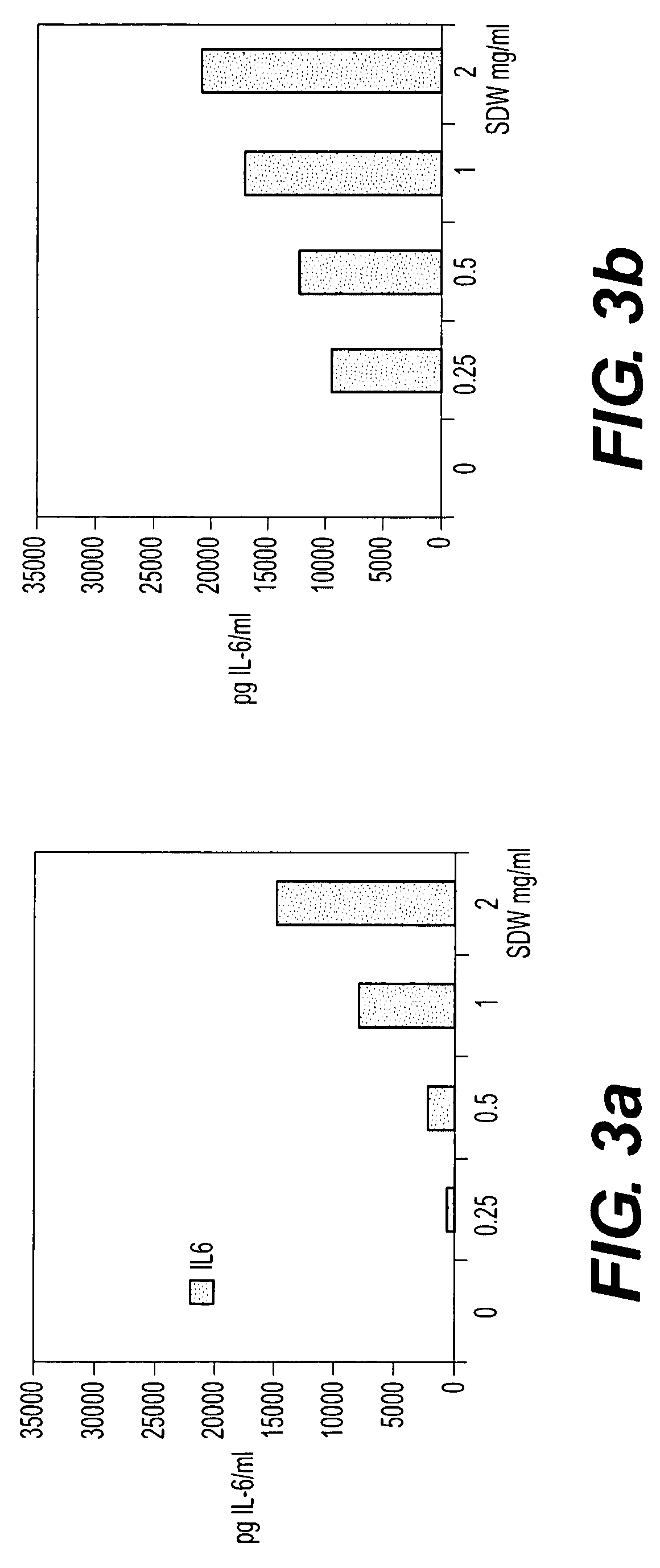
Patents
Literature
67 results about "Urinary tract disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
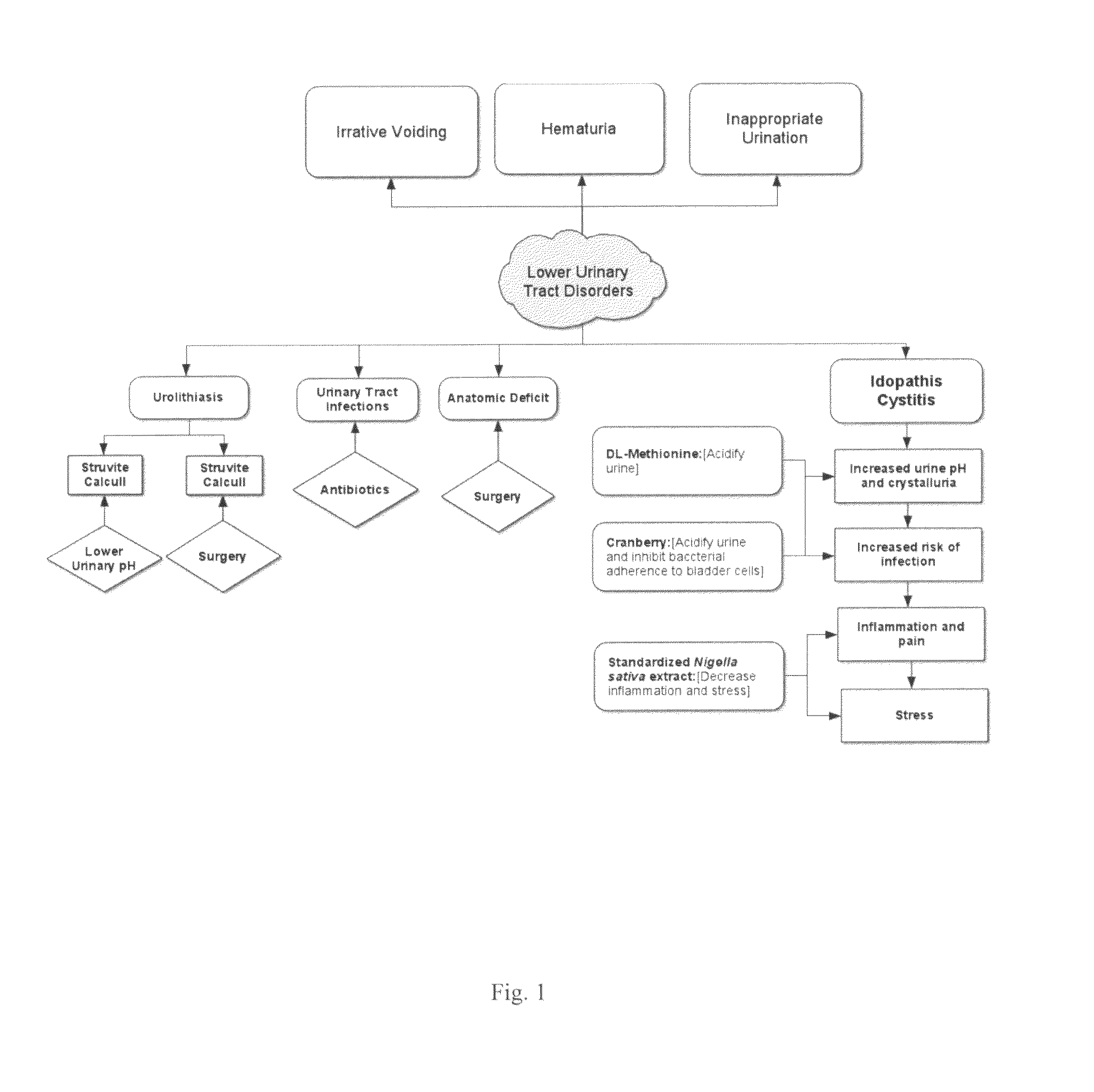
Condition in which there is a deviation from or interruption of the normal structure or function of the urinary tract.
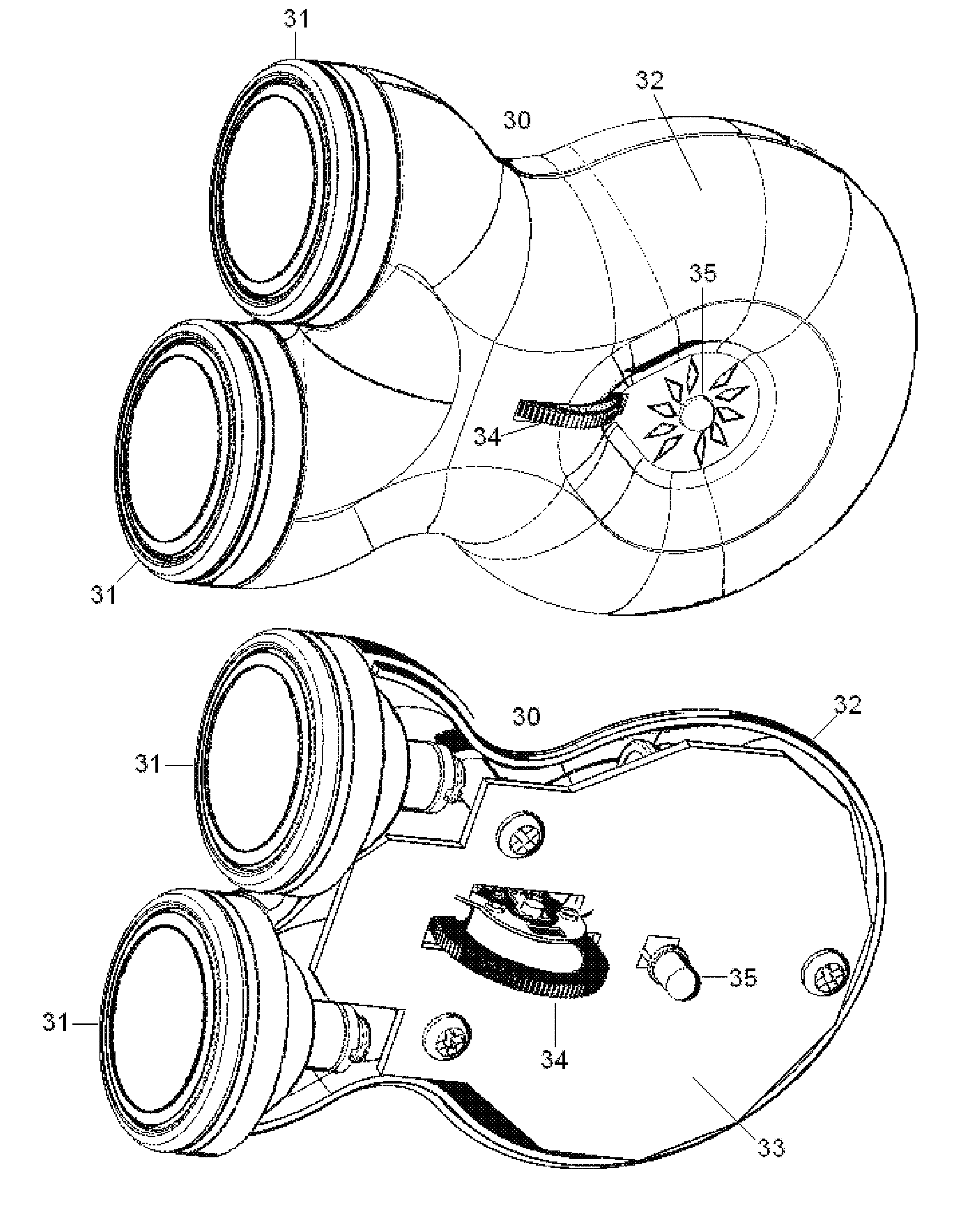
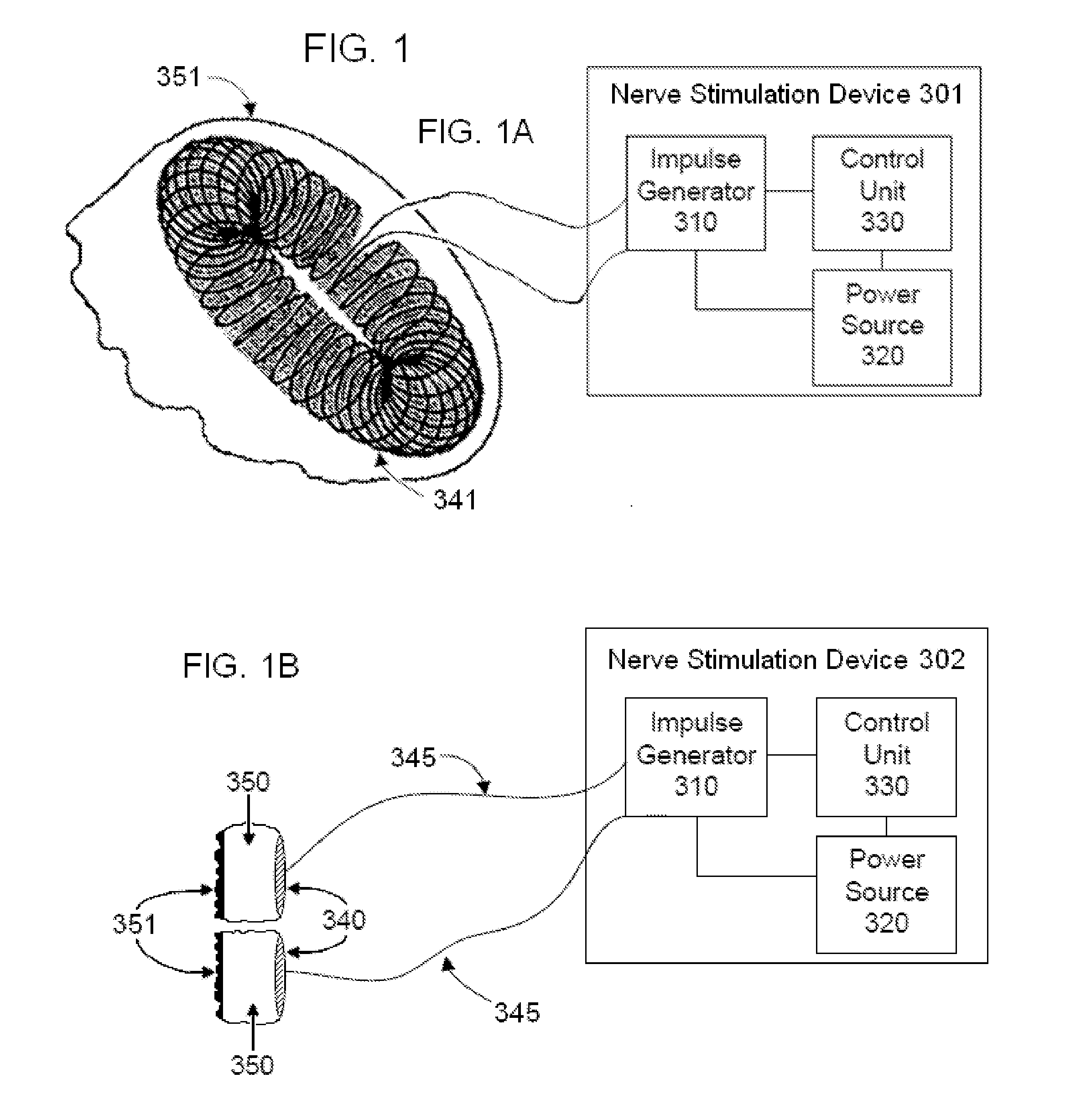
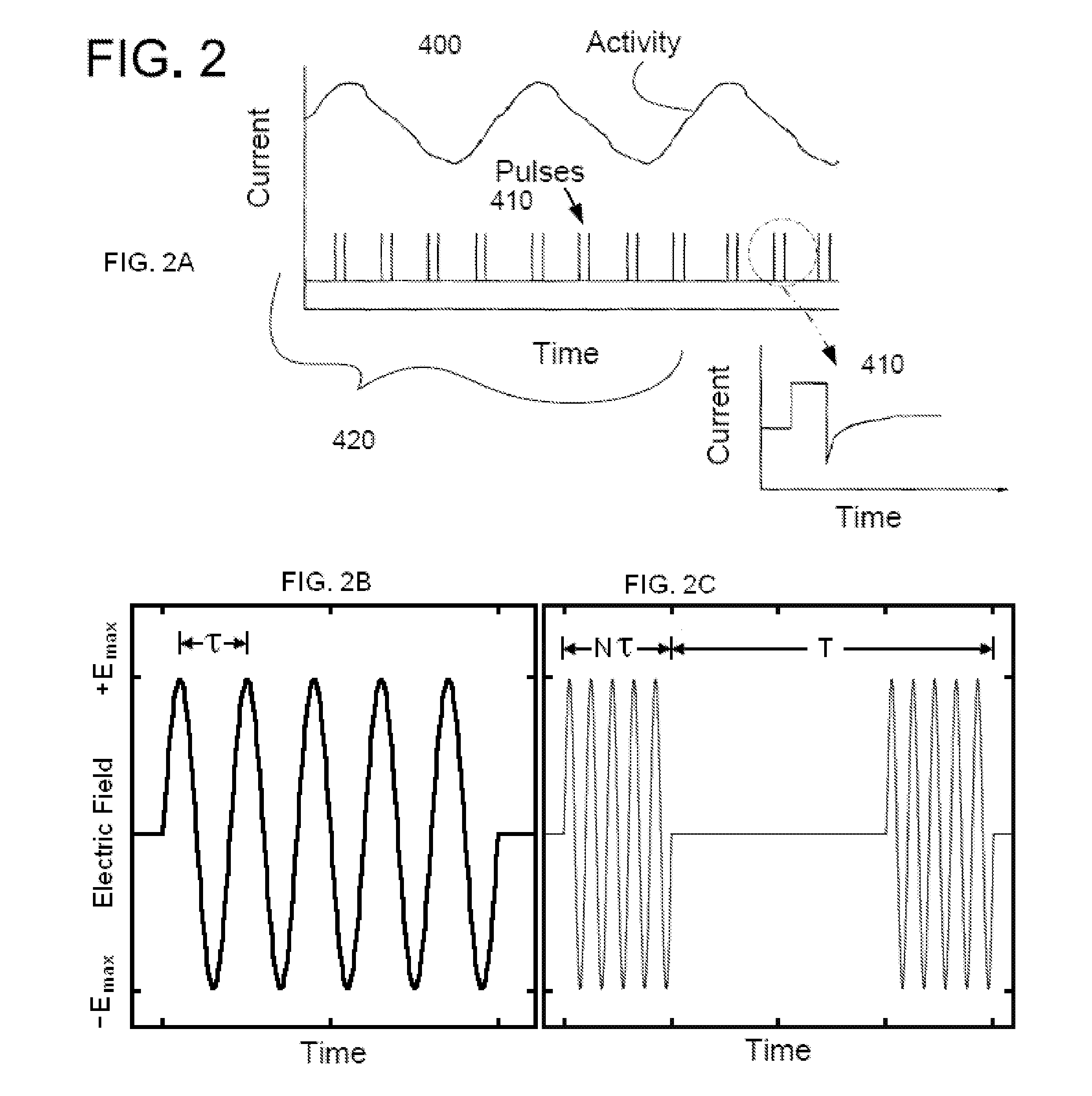
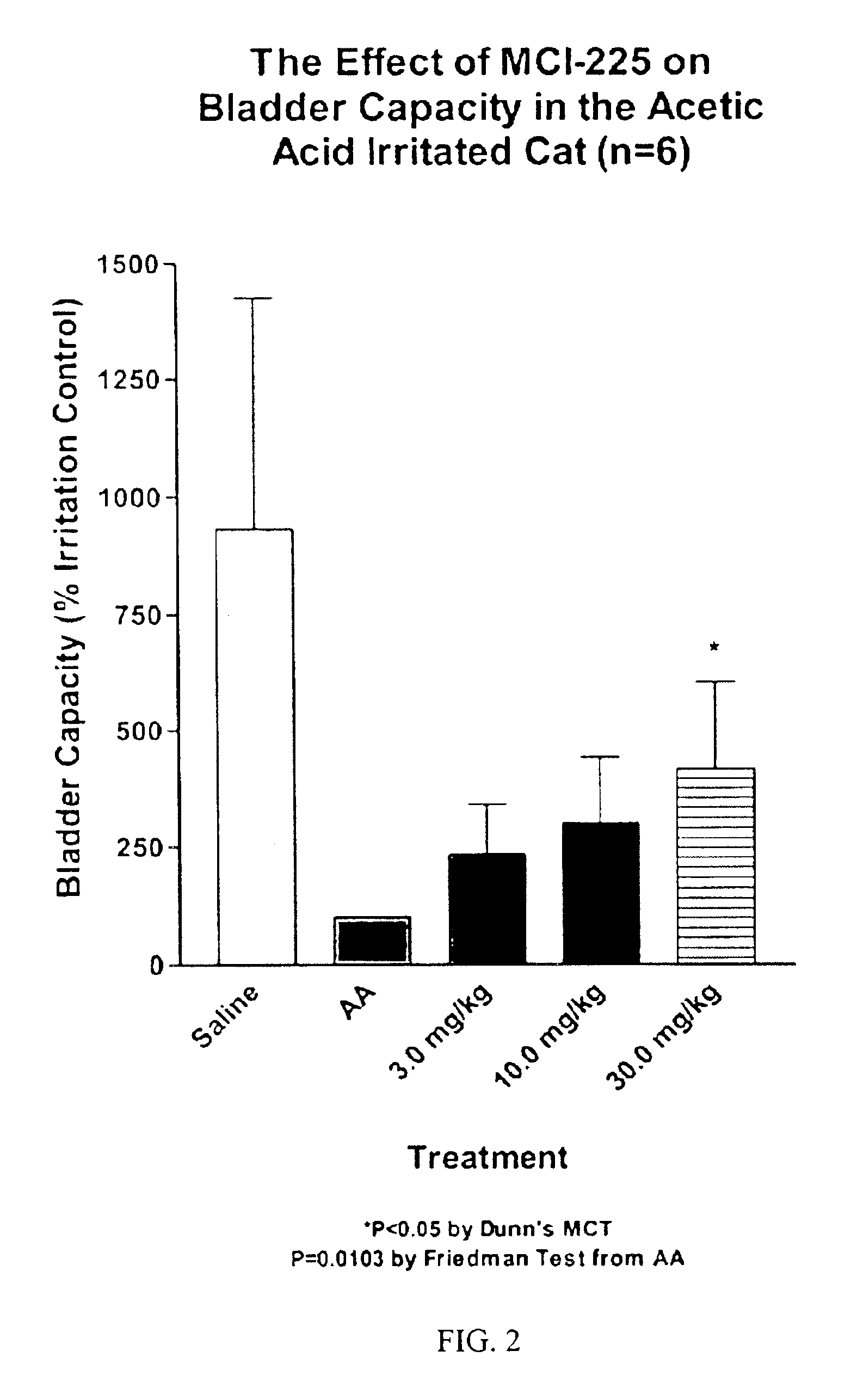
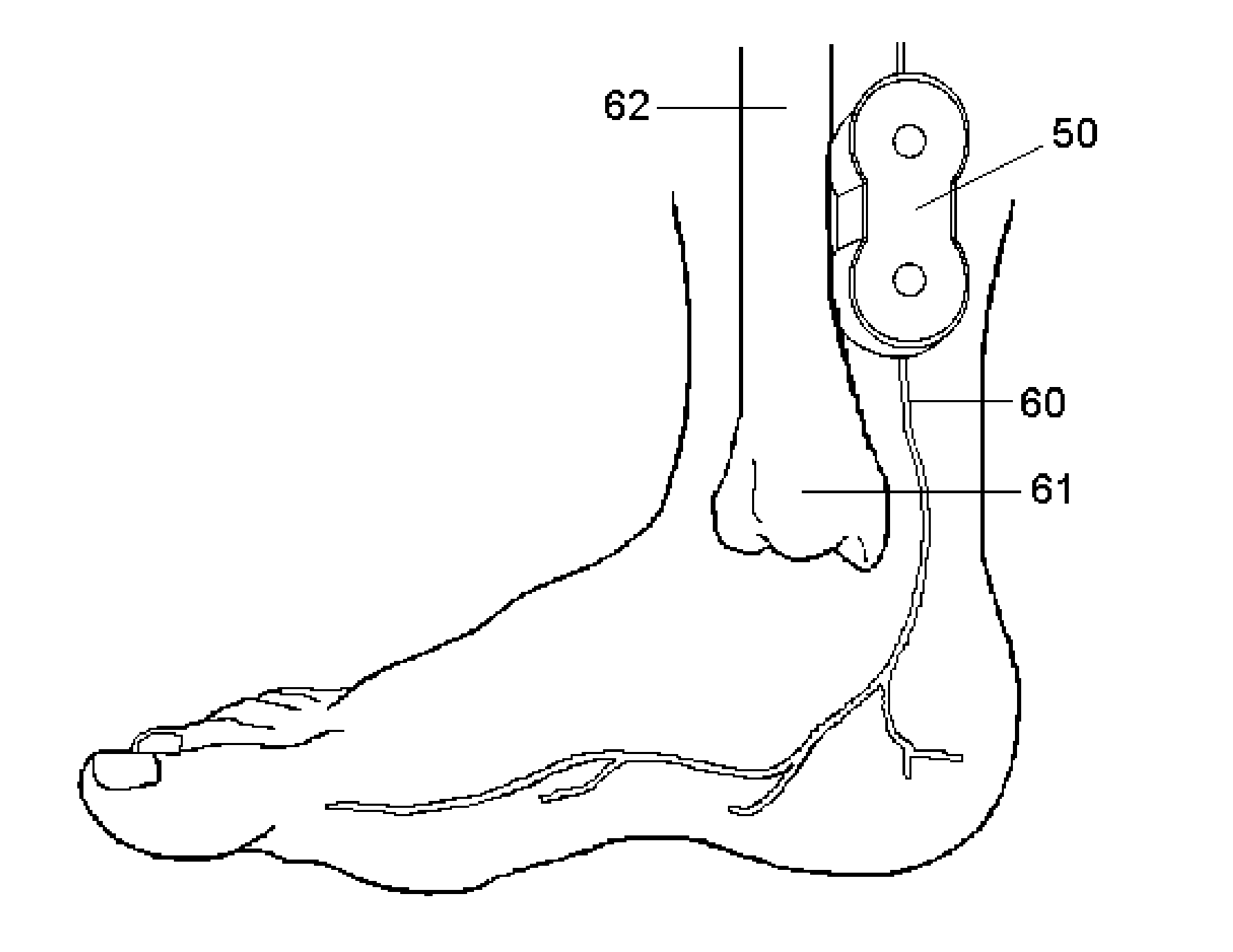
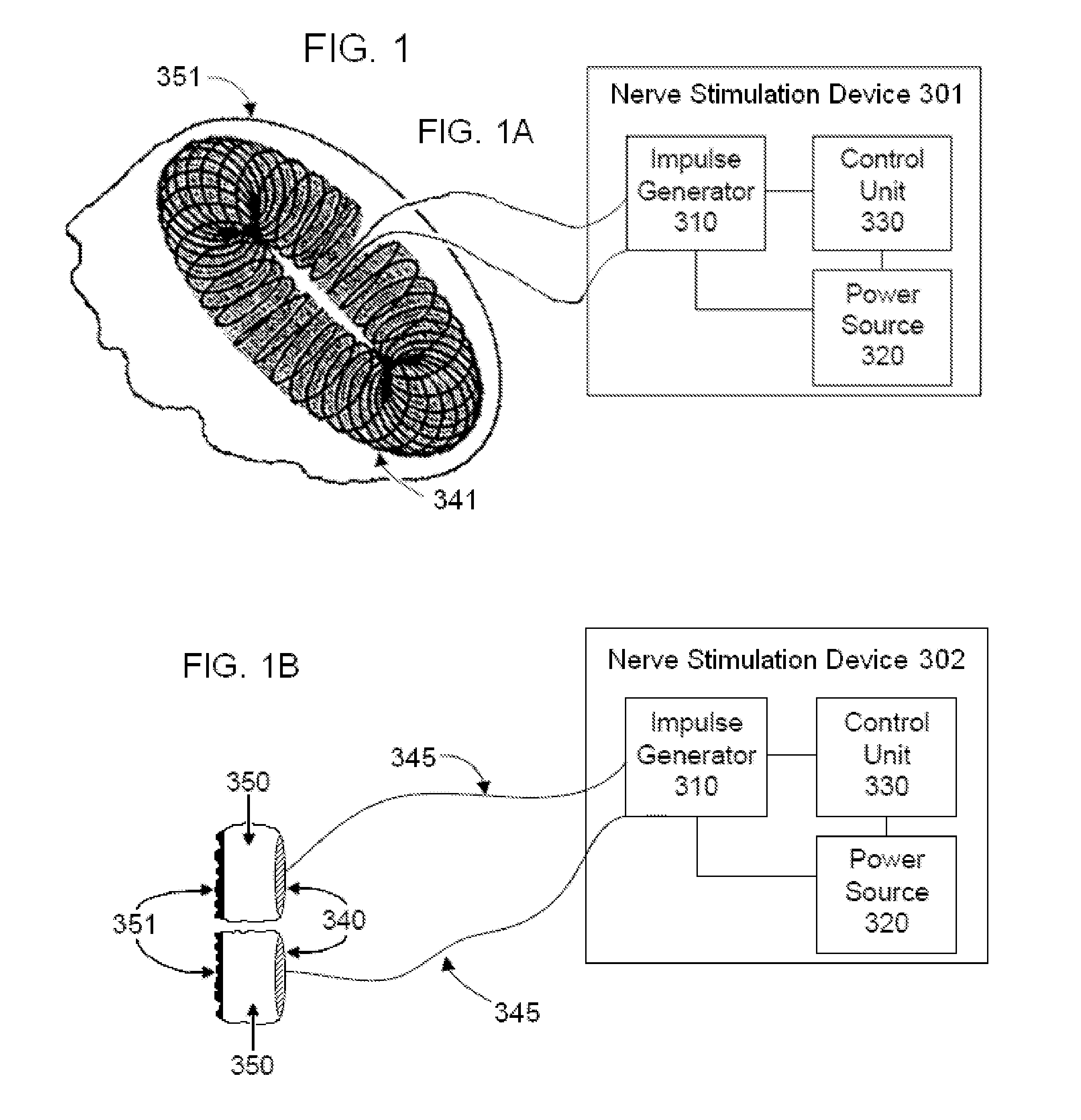
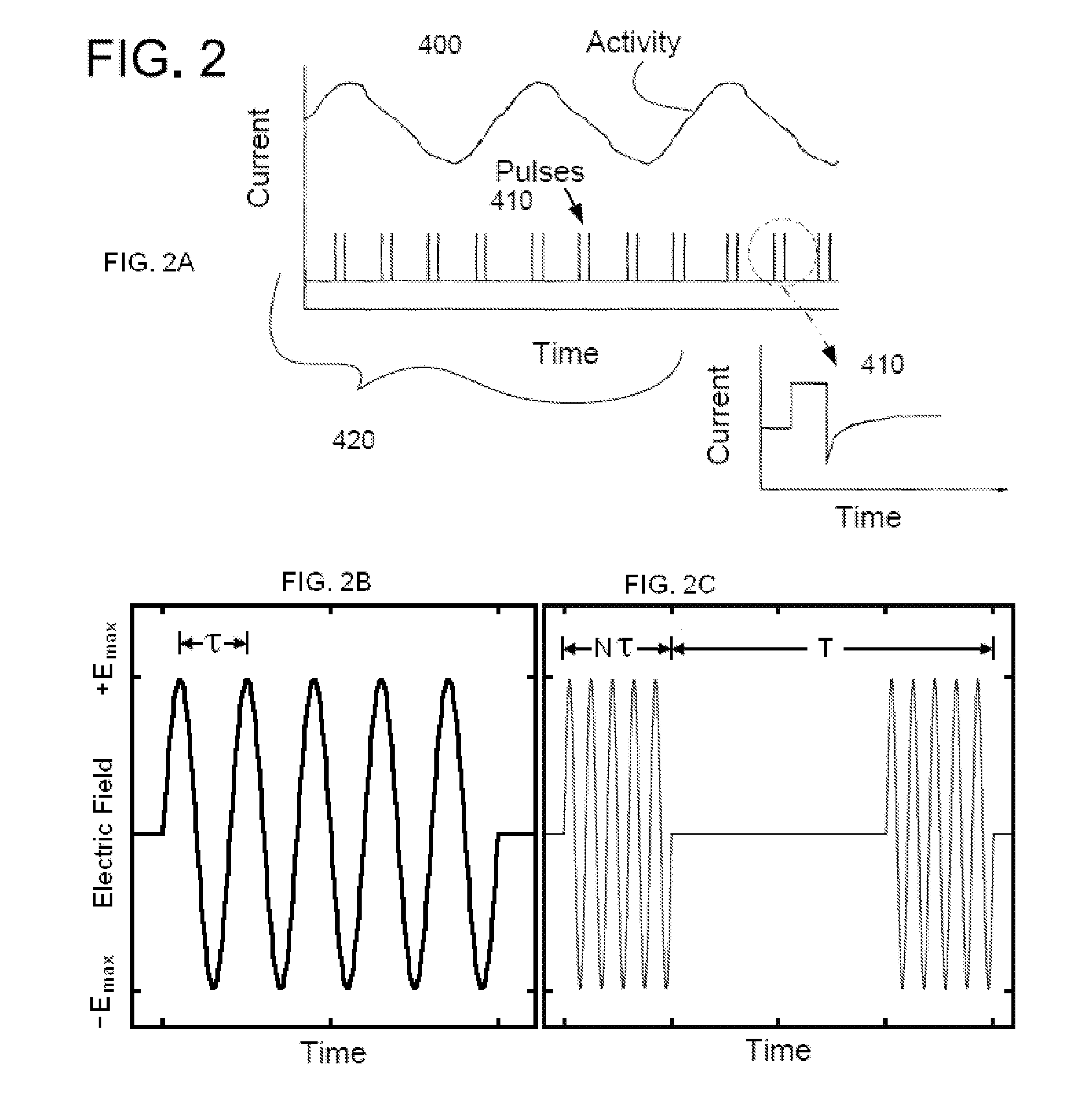
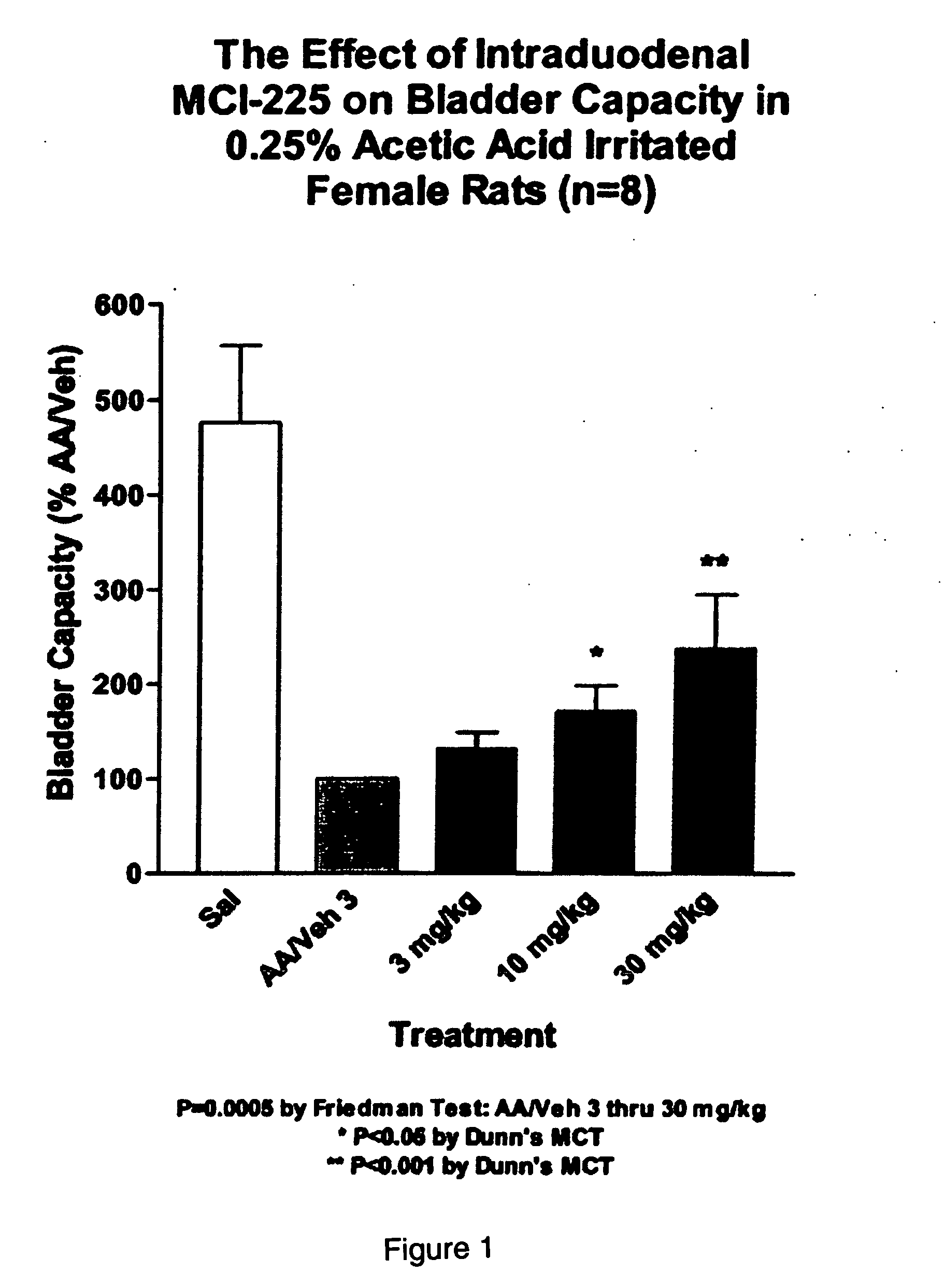
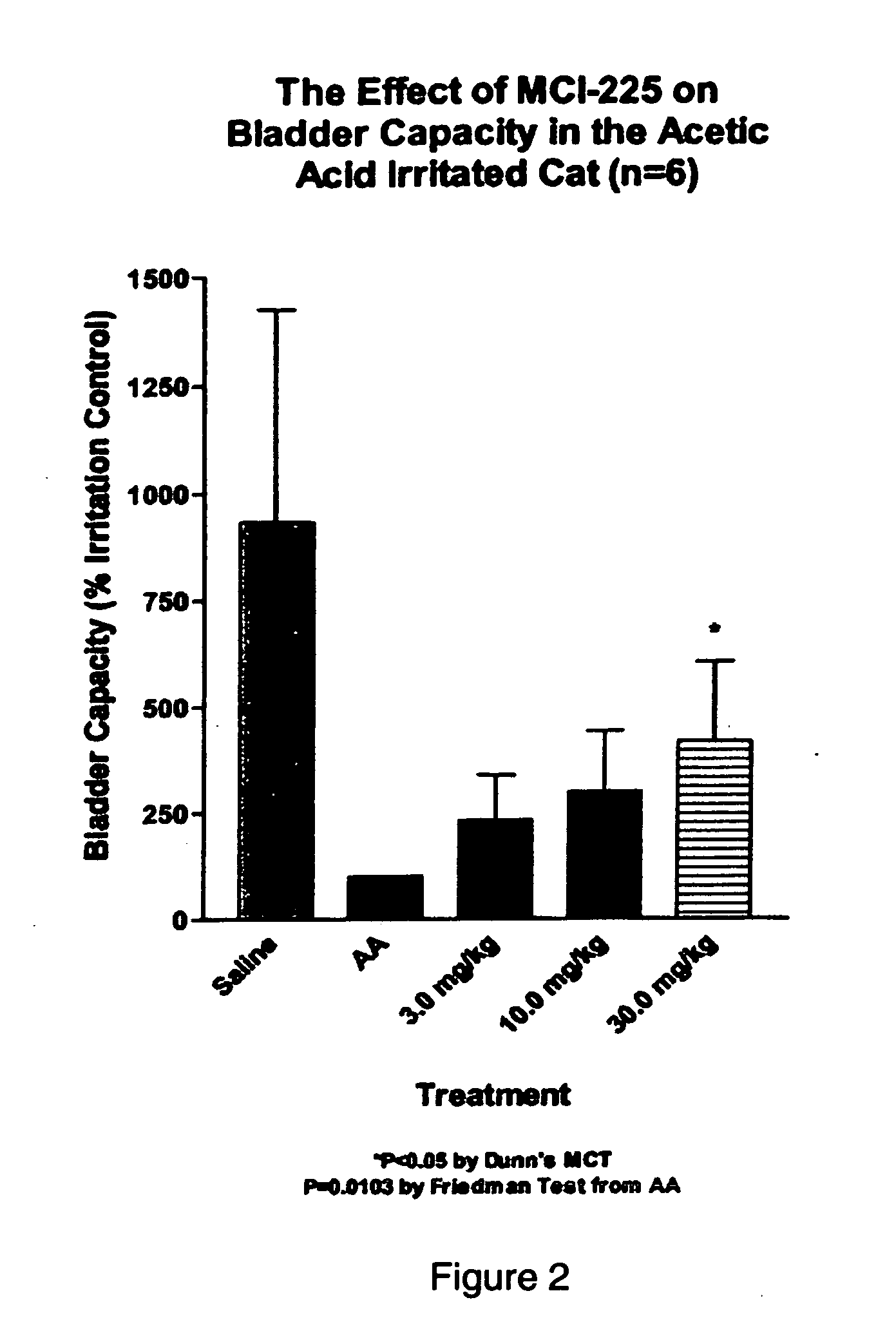
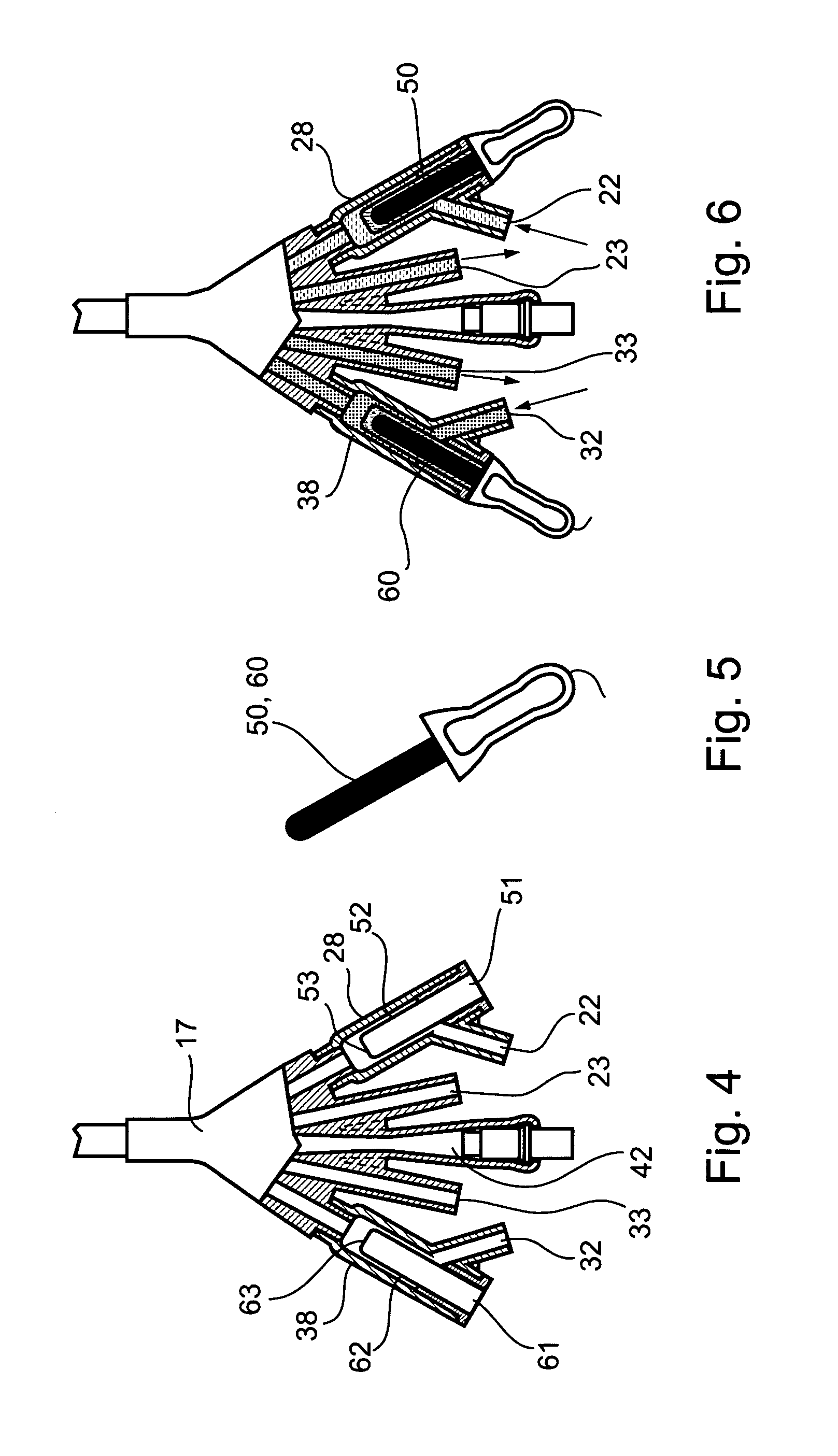
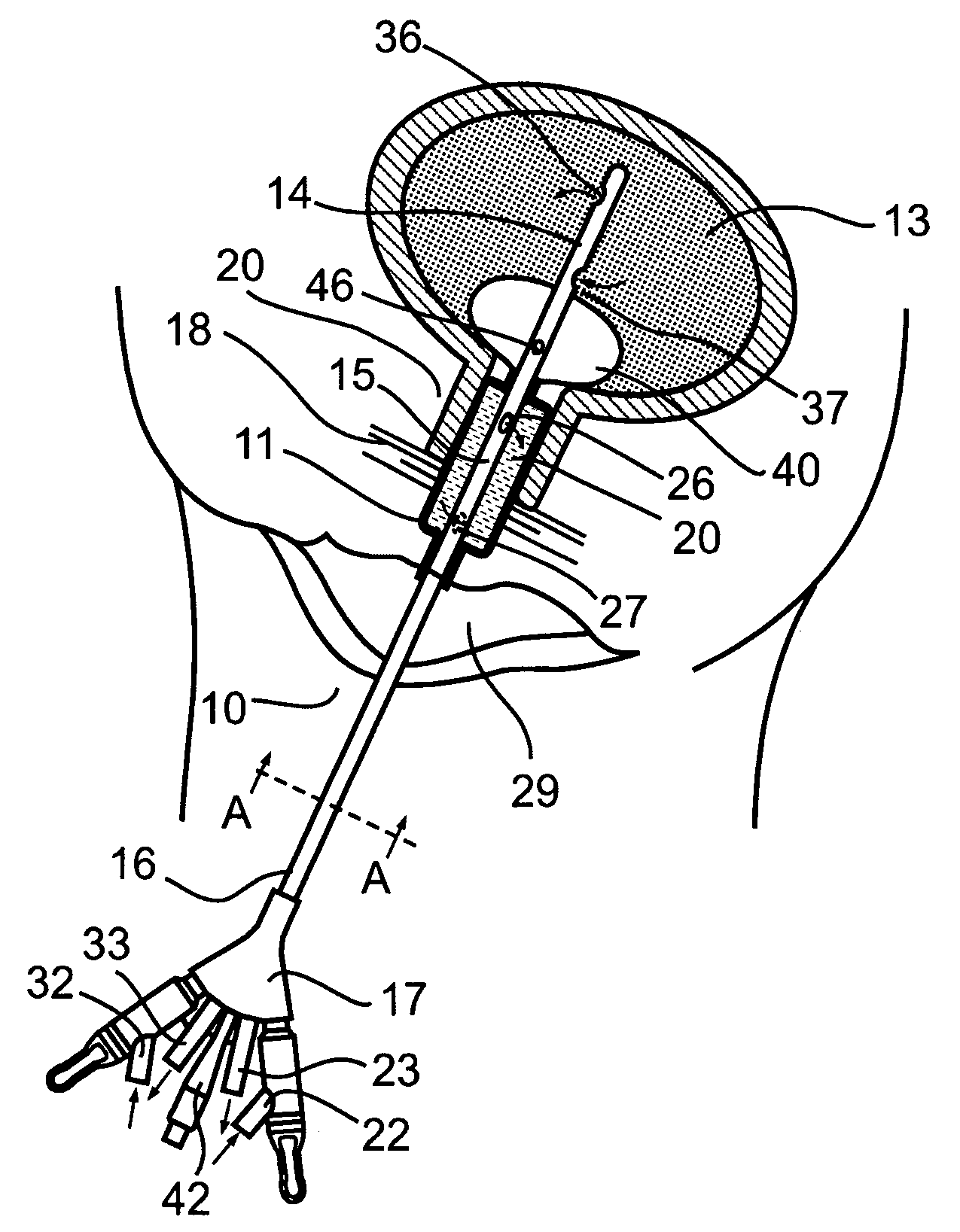
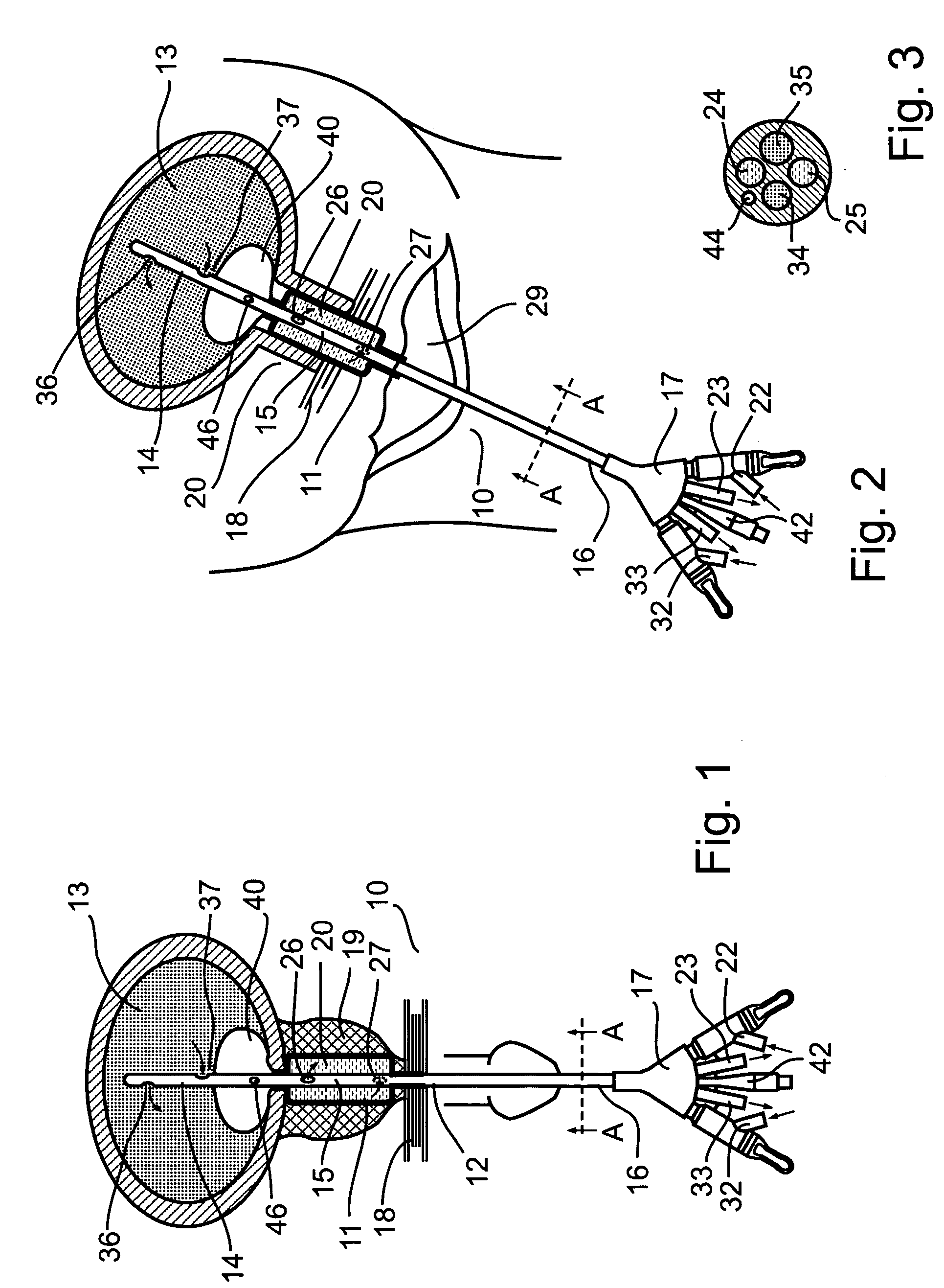
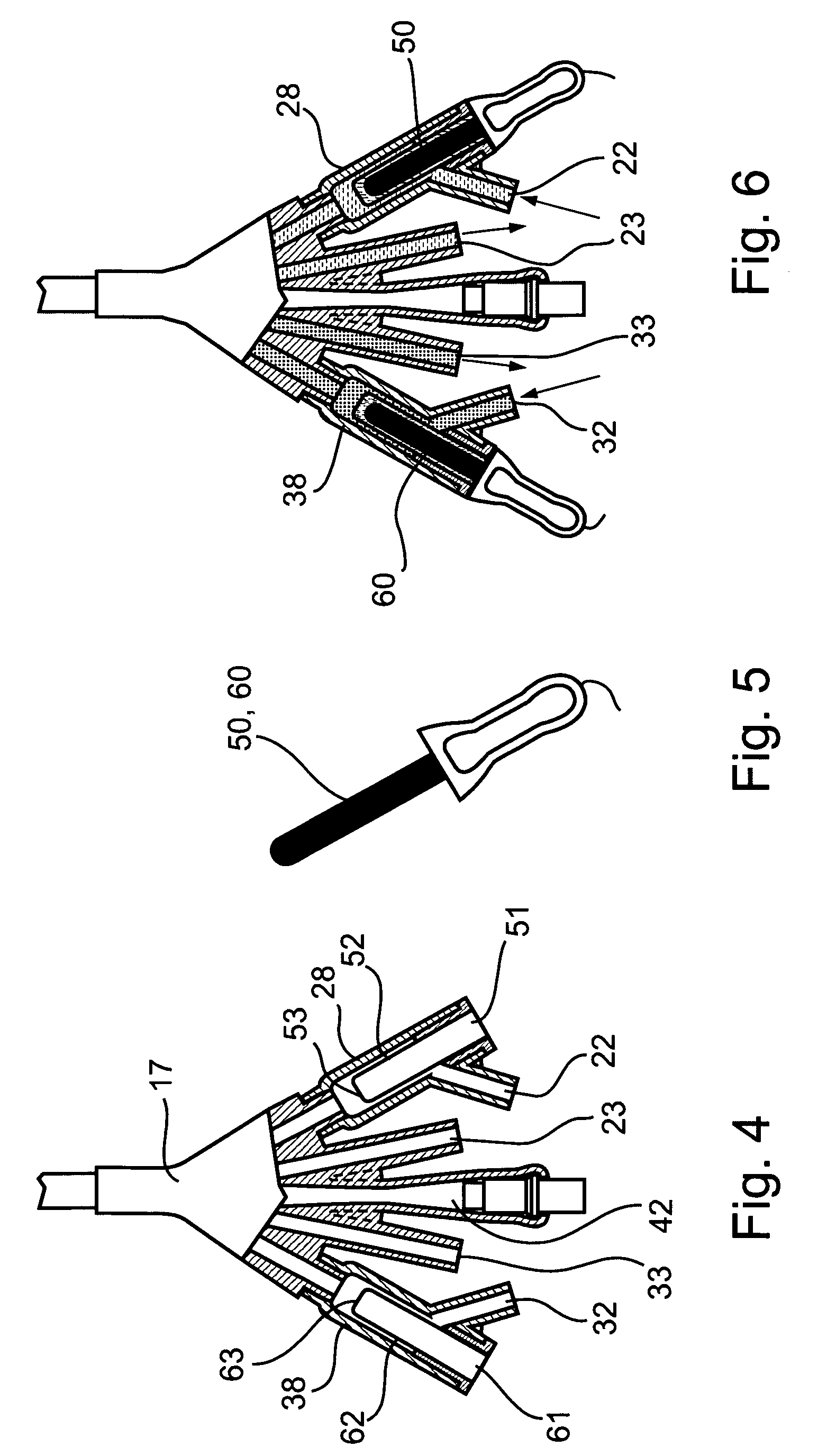
Non-invasive electrical and magnetic nerve stimulators used to treat overactive bladder and urinary incontinence
Transcutaneous electrical and magnetic nerve stimulation devices are disclosed, along with methods of treating lower urinary tract disorders using energy that is delivered noninvasively by the devices. The disorders comprise overactive bladder, urge incontinence, stress, incontinence, urge frequency, non-obstructive urinary retention and interstitial cystitis / painful bladder syndrome. In preferred embodiments of the disclosed methods, a posterior tibial nerve of a patient is stimulated non-invasively. Methods are disclosed for selecting protocol parameters for a nerve stimulation session for treating each individual patient, wherein modules of the bladder of the patient are represented as coupled non-linear oscillators.
Owner:ELECTROCORE
Method of treating lower urinary tract disorders
The invention relates to a method of treating at least one symptom of a lower urinary tract disorder in a subject in need of treatment wherein the symptom is selected from the group consisting of urinary frequency, urinary urgency, urinary urge incontinence, nocturia and enuresis. The method comprises administering to a subject in need of treatment a therapeutically effective amount of a compound that has 5-HT3 receptor antagonist activity and NorAdrenaline Reuptake Inhibitor (NARI) activity. The invention further relates to a method of treating at least one symptom of a lower urinary tract disorder in a subject in need of treatment wherein the symptom is selected from the group consisting of urinary frequency, urinary urgency, urinary urge incontinence, nocturia and enuresis, comprising coadministering to said subject a first amount of a 5-HT3 antagonist and a second amount of a NARI, wherein the first and second amounts together comprise a therapeutically effective amount or are each present in a therapeutically effective amount.
Owner:EDUSA PHARMA
Swollen hydrogel for sphincter augmentation
InactiveUS7049346B1Lowered IFsReduce impactPowder deliverySurgical adhesivesSimple Organic CompoundsSphincter
A physiologically acceptable composition comprises a plurality of physiologically acceptable hydrogel particles which have been swollen in a water solution containing a low molecular weight water soluble organic compound, the solution having the ability to swell the particles. The concentration of the organic compound is such that the resulting particles can be inserted, without use of a carrier liquid utilizing a hand driven hypodermic syringe. The swollen hydrogel particles are substantially insoluble in body fluids. The composition is useful for treating urinary tract disorders.
Owner:MENLO CARE INC JOHNSON & JOHNSON
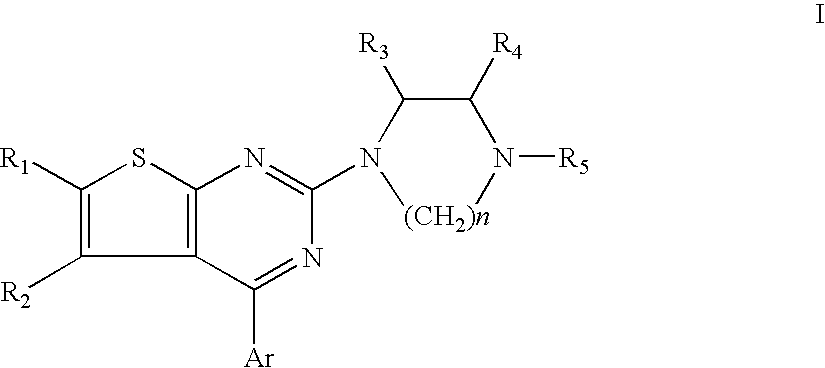
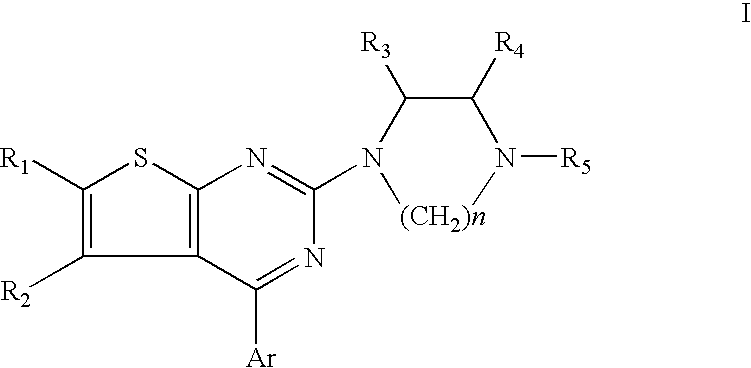
Nitrogen-containing heterocyclic compound and use thereof
ActiveUS20090156572A1Easy to optimizeLow toxicityBiocideNervous disorderIntestinal tract diseasesDisease
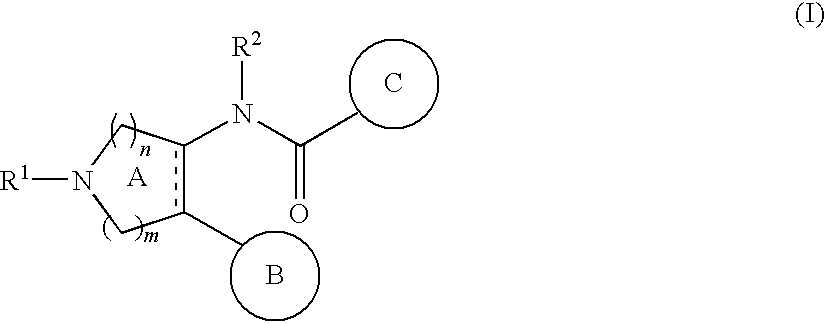
The present invention relates to a compound represented by the formulawherein ring A is a nitrogen-containing heterocycle optionally further having substituent(s), ring B is an aromatic ring optionally having substituent(s), ring C is a cyclic group optionally having substituent(s), R1 is a hydrogen atom, a hydrocarbon group optionally having substituent(s), an acyl group, a heterocyclic group optionally having substituent(s) or an amino group optionally having substituent(s), R2 is an optionally halogenated C1-6 alkyl group, m and n are each an integer of 0 to 5, m+n is an integer of 2 to 5, and is a single bond or a double bond, or a salt thereof and the like. Since the compound has a superior tachykinin receptor antagonistic action, and is useful as an agent for the prophylaxis or treatment of various diseases such as lower urinary tract diseases, gastrointestinal diseases, central nervous system diseases and the like.
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for the treatment of urinary incontinence
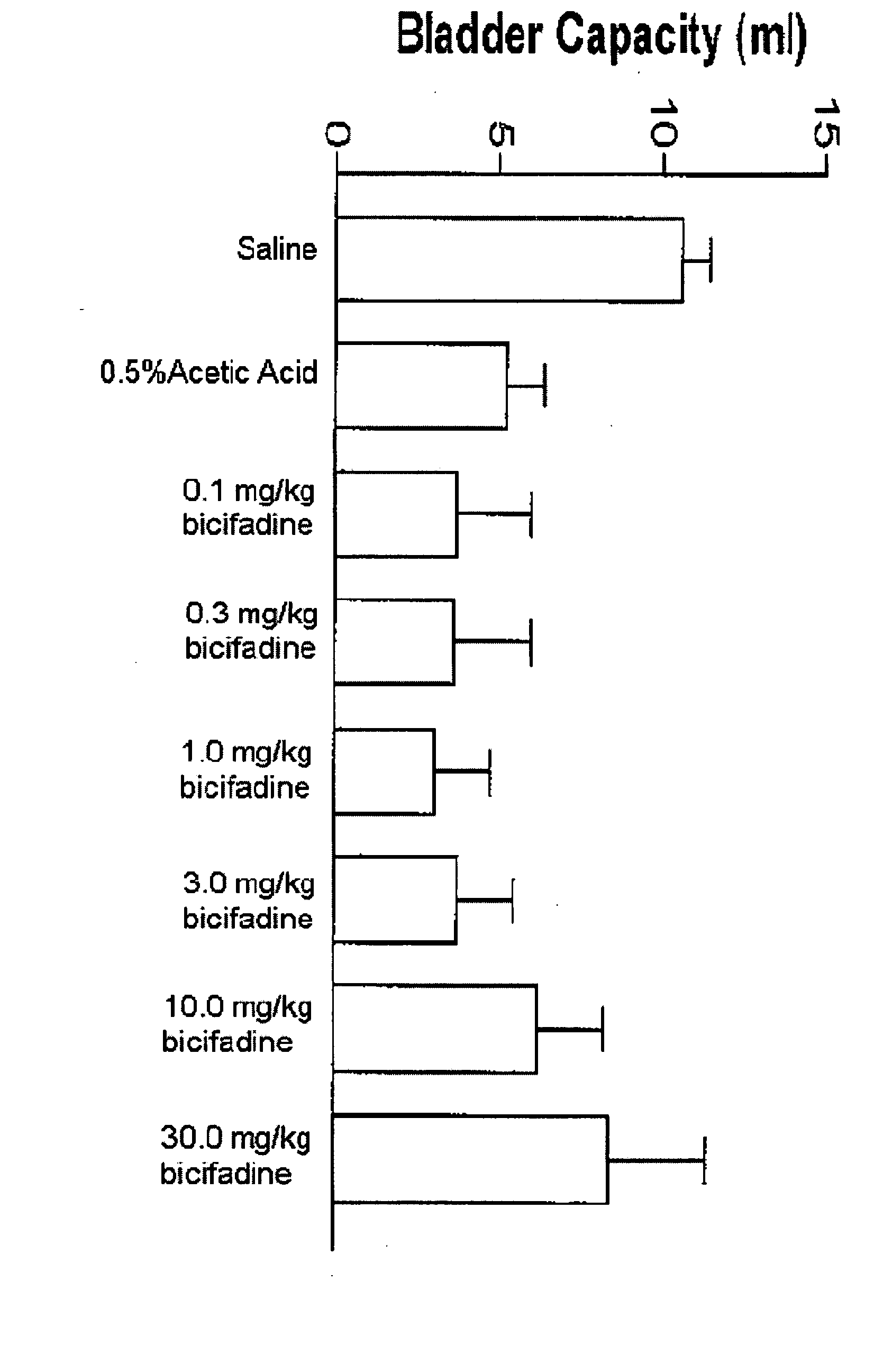
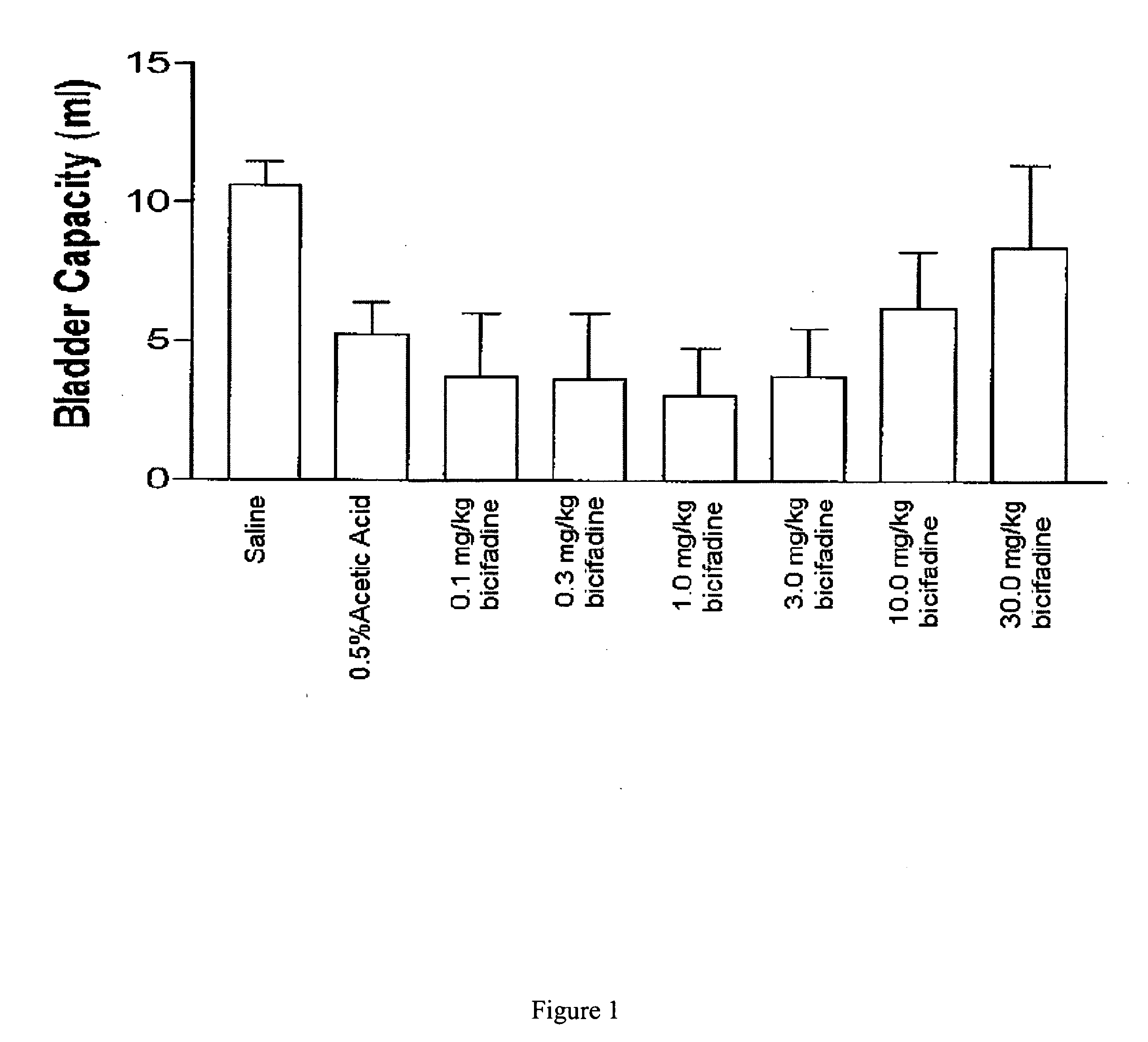
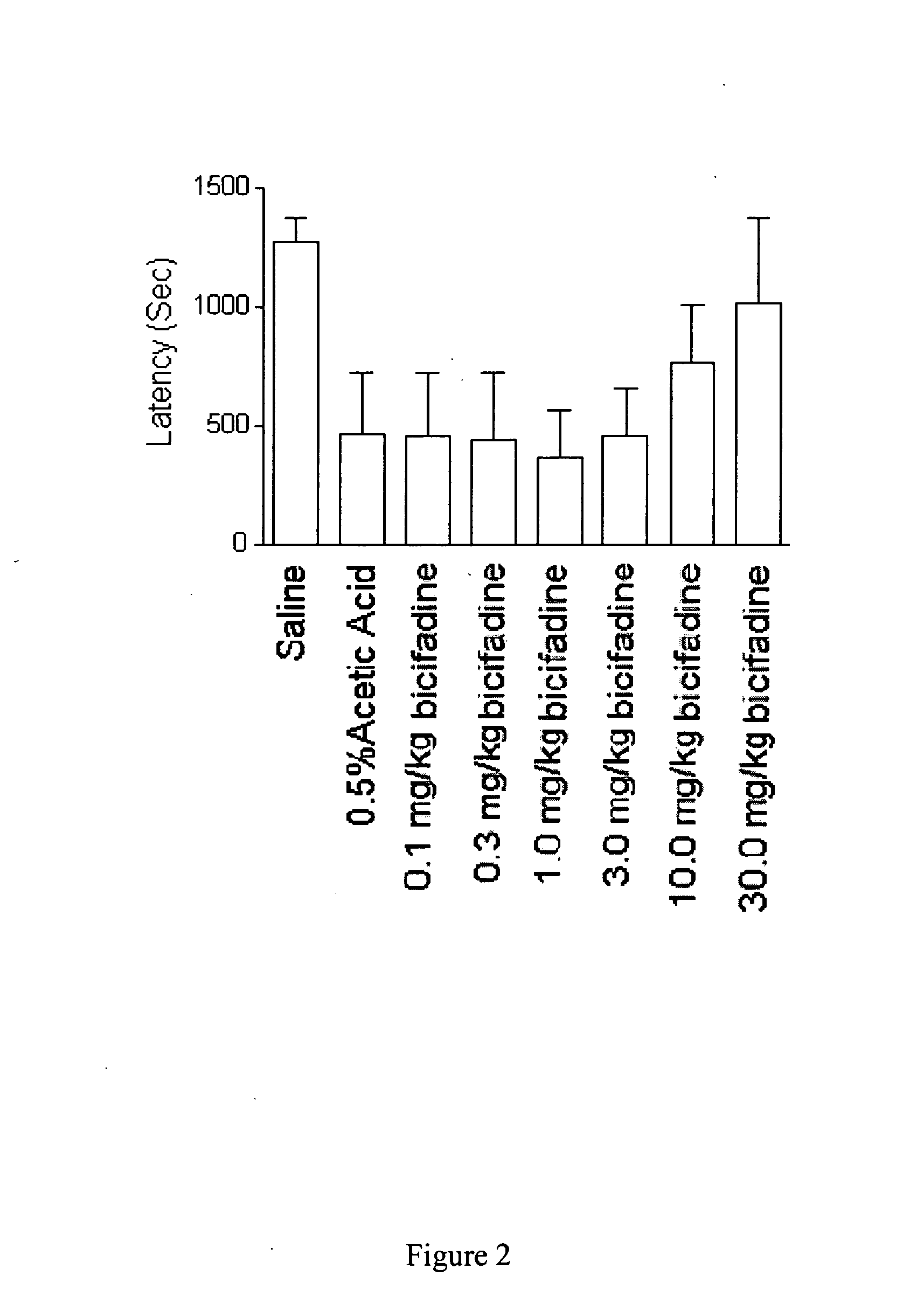
InactiveUS20080009538A1Lower urinary tract disordersBiocideUrinary disorderBicifadineNeuropathic bladder
Methods and compositions containing bicifadine are provided for the prevention and treatment of lower urinary tract disorders in mammalian subjects. The methods and compositions may be used to prevent or treat urinary incontinence, urinary urgency, nocturia, and enuresis associated with neurogenic and non-neurogenic overactive bladder, interstitial cystitis, prostatitis, prostadynia, and benign prostatic hyperplasia, among other conditions. Additional compositions and methods are provided which employ bicifadine in combination with a second anti-incontinence agent, or a different therapeutic agent to yield more effective anti-incontinence treatment tools, and / or dual activity therapeutic methods and formulations useful to prevent or reduce urinary incontinence and one or more additional symptoms such as urinary urgency, overflow, frequency, or pain in mammalian subjects.
Owner:DOV PHARMA
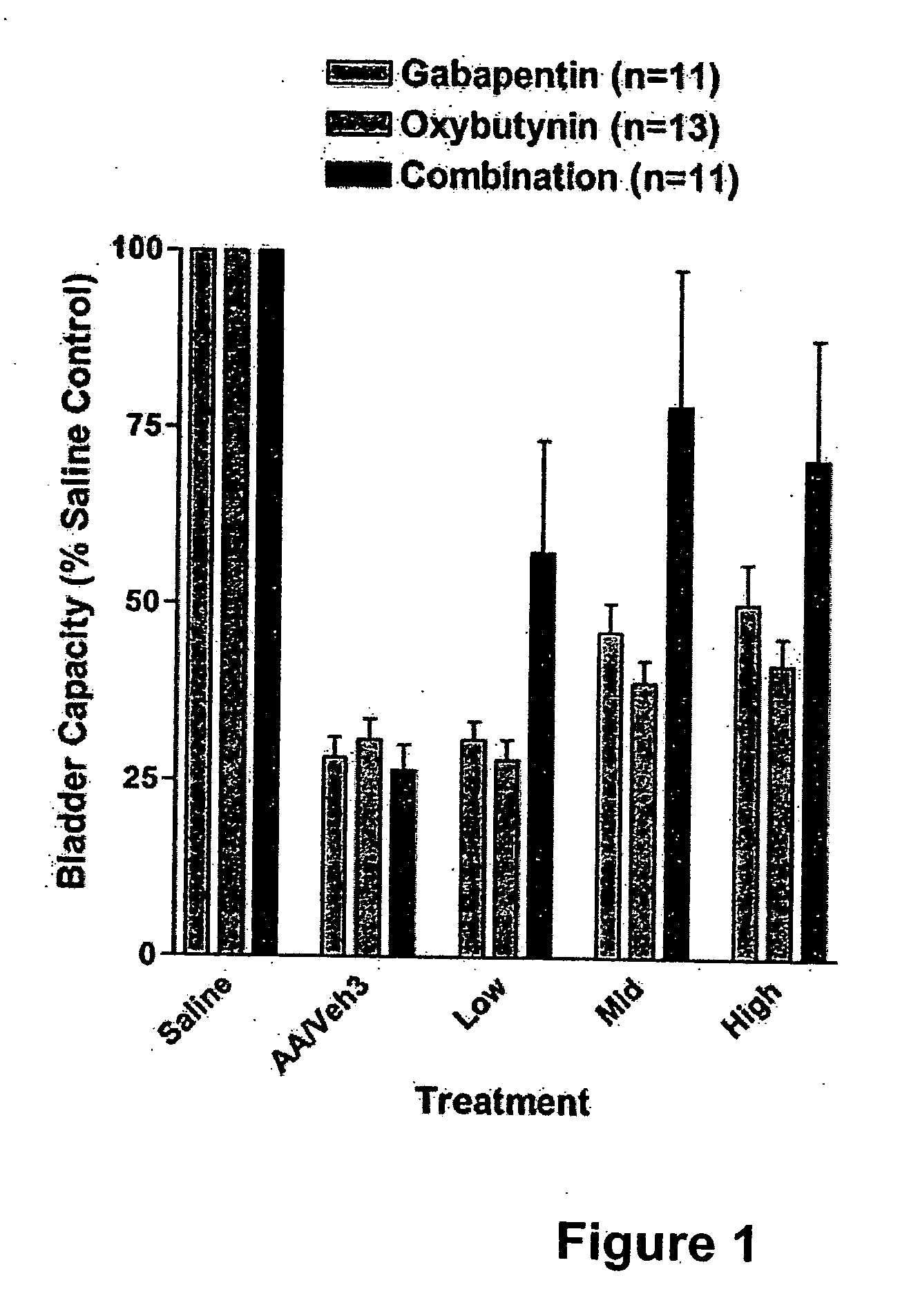
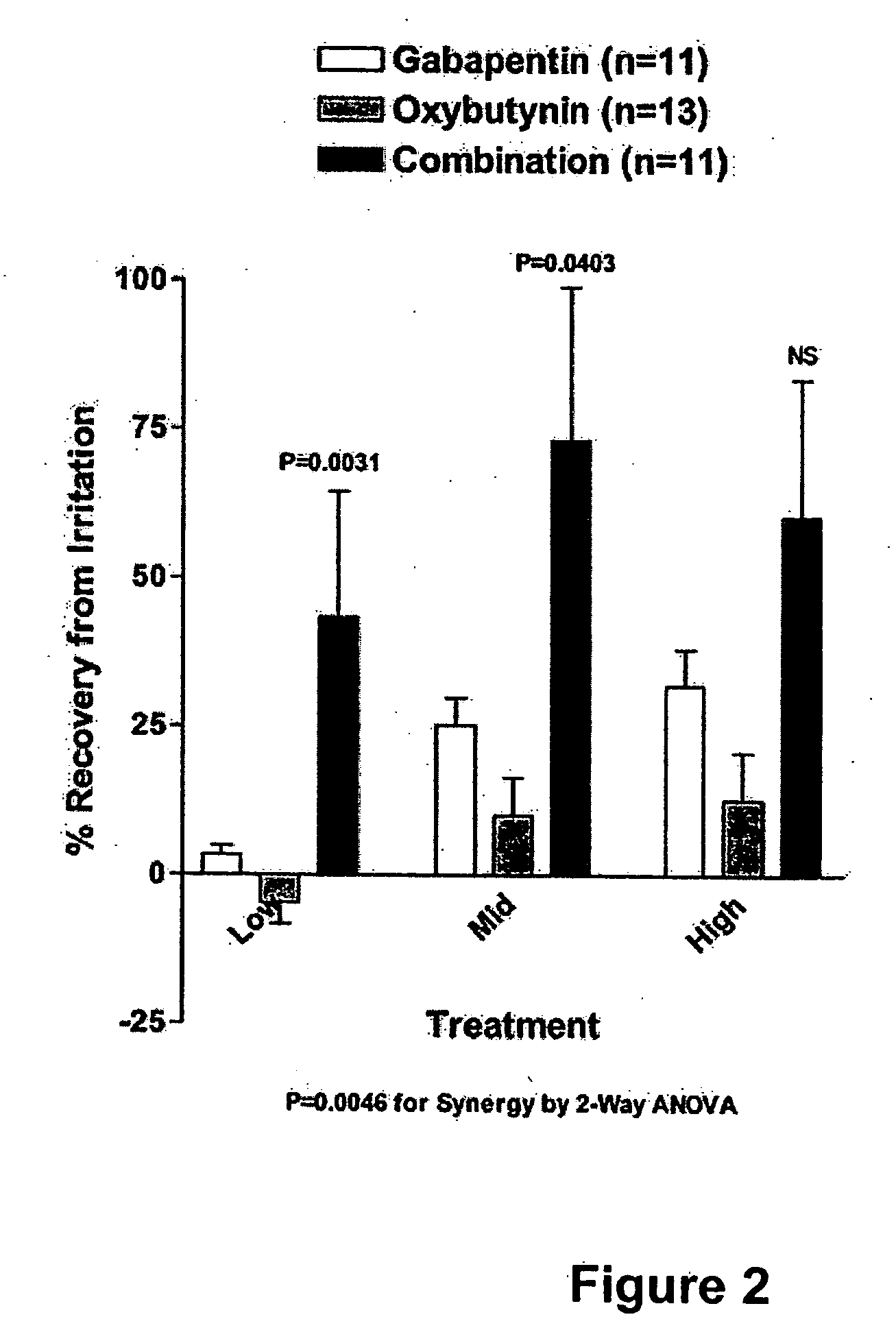
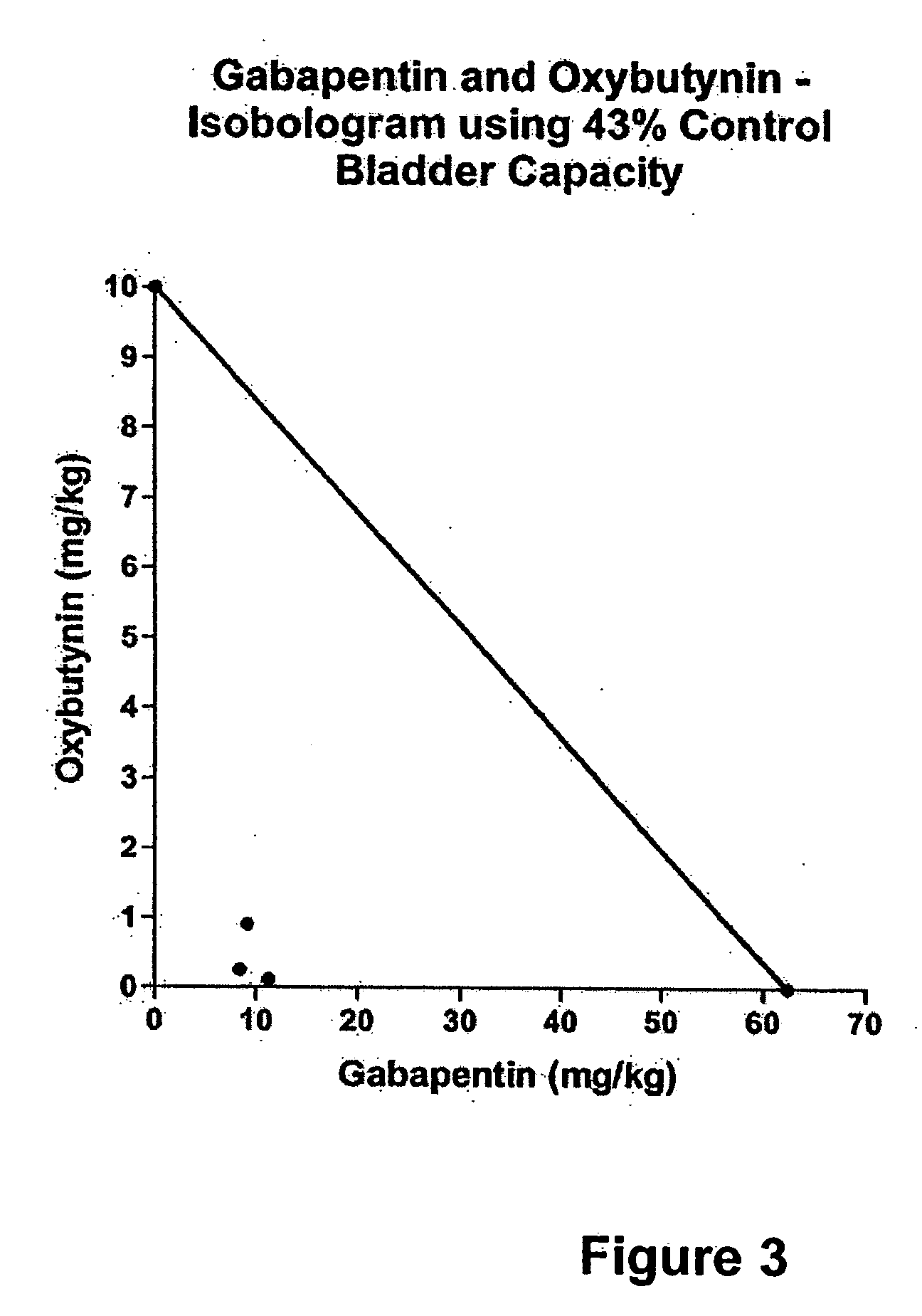
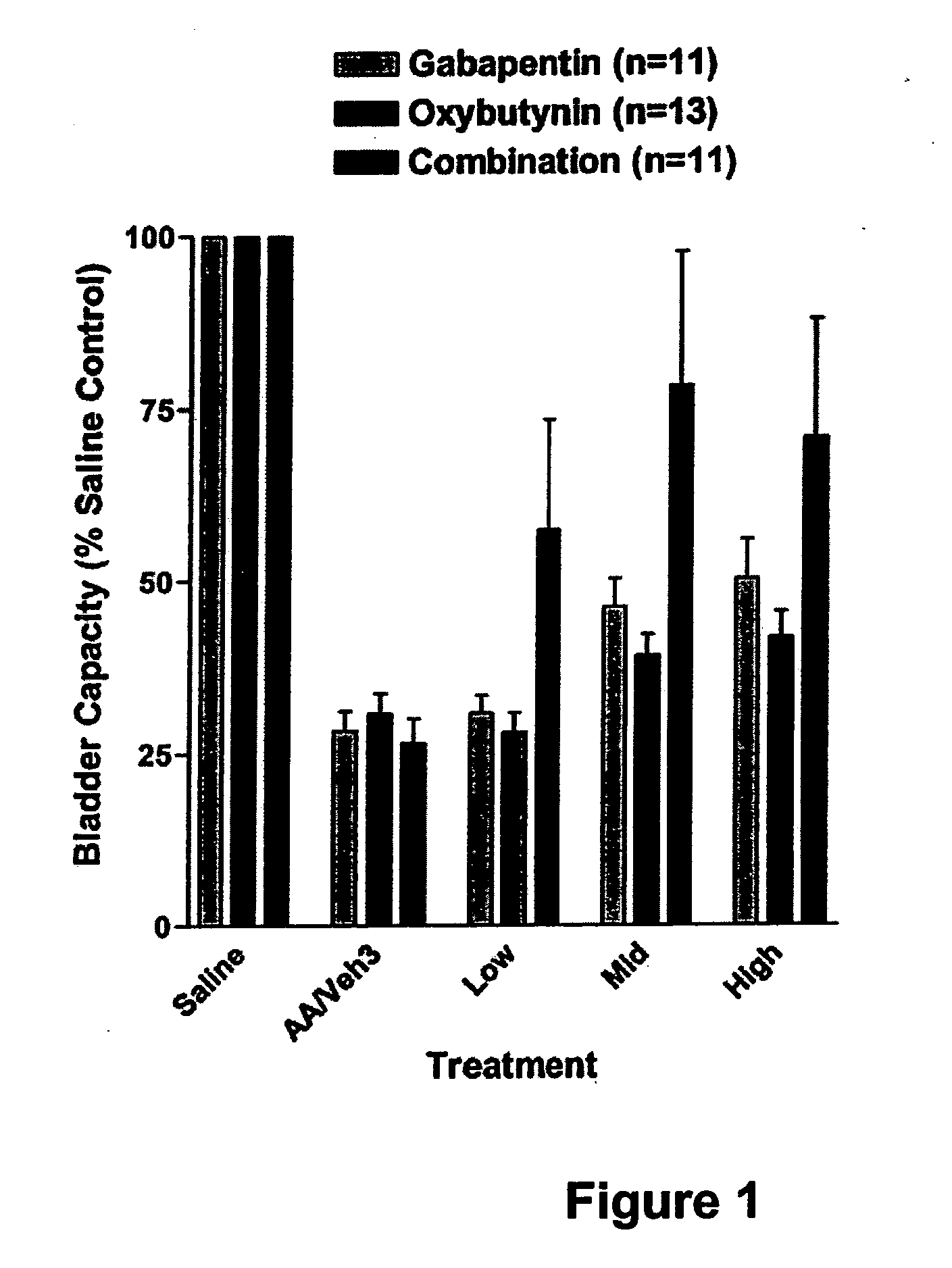
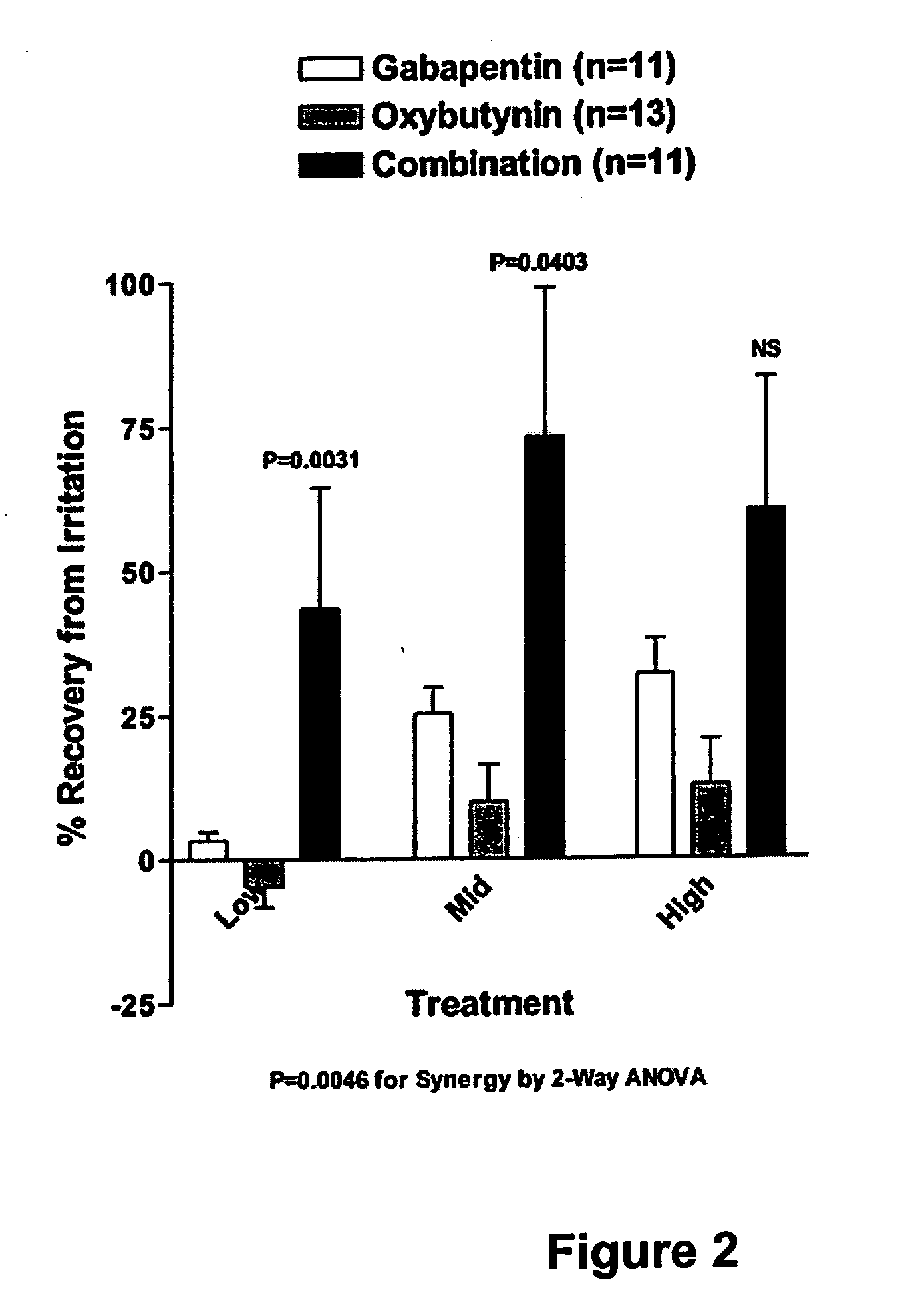
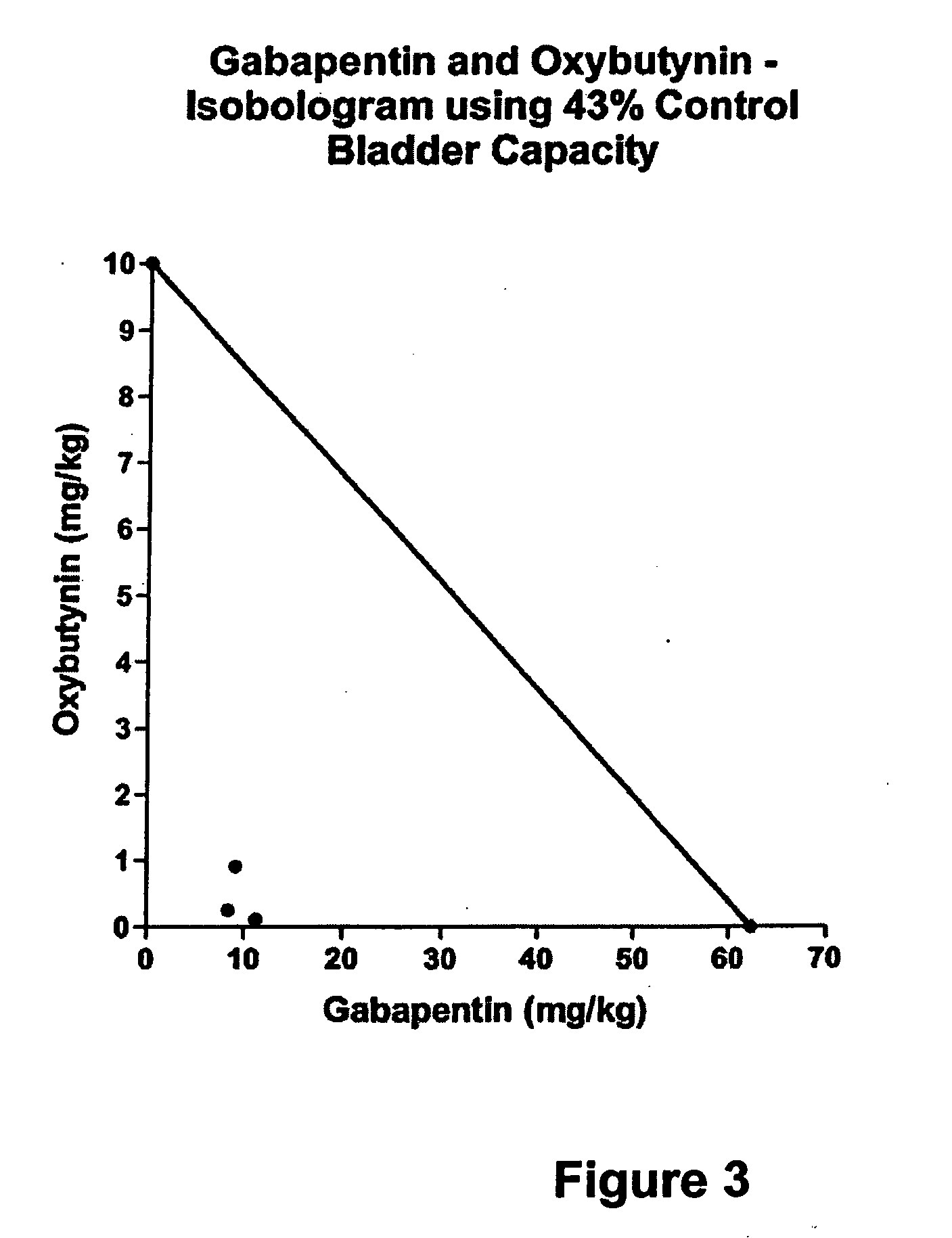
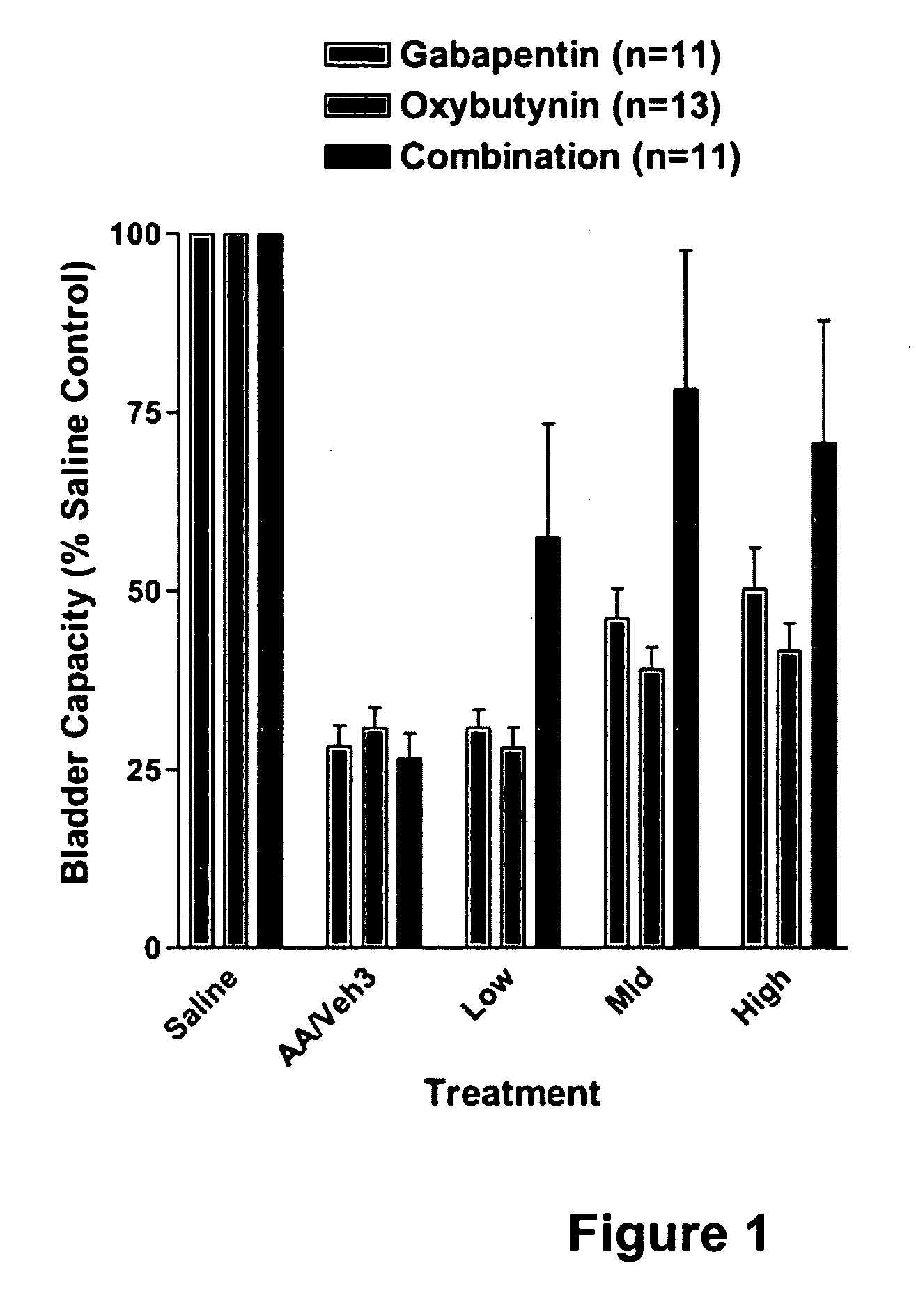
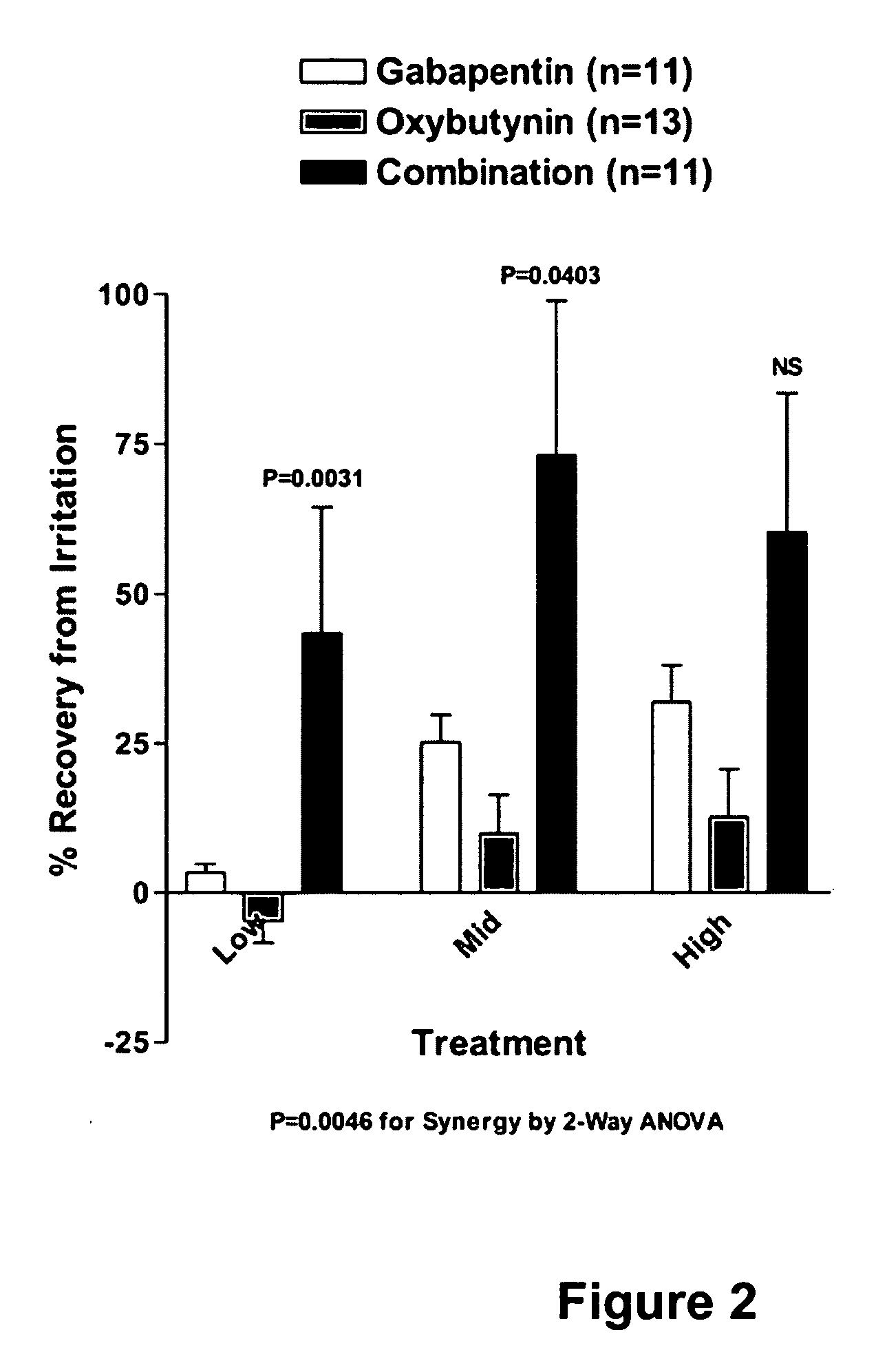
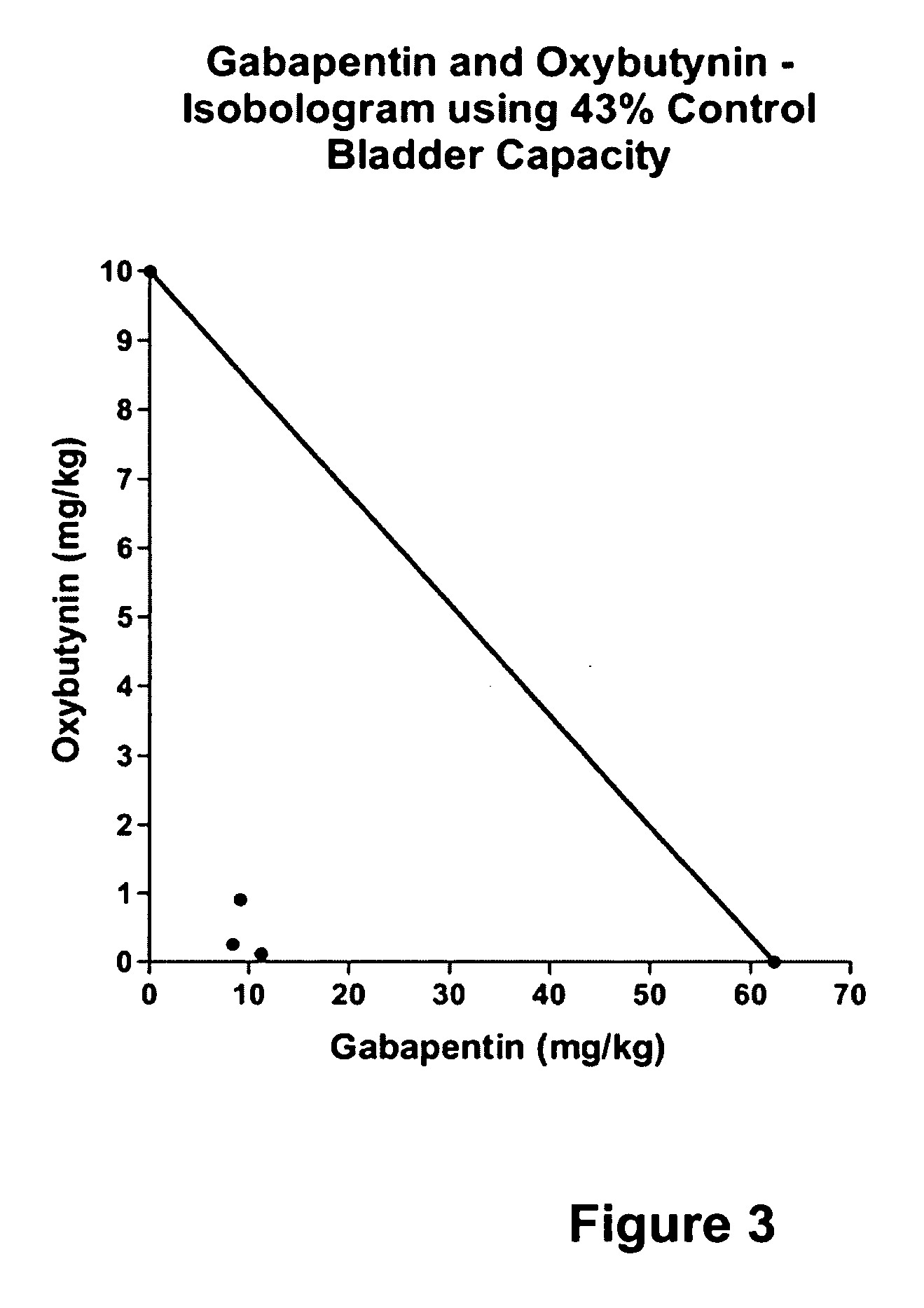
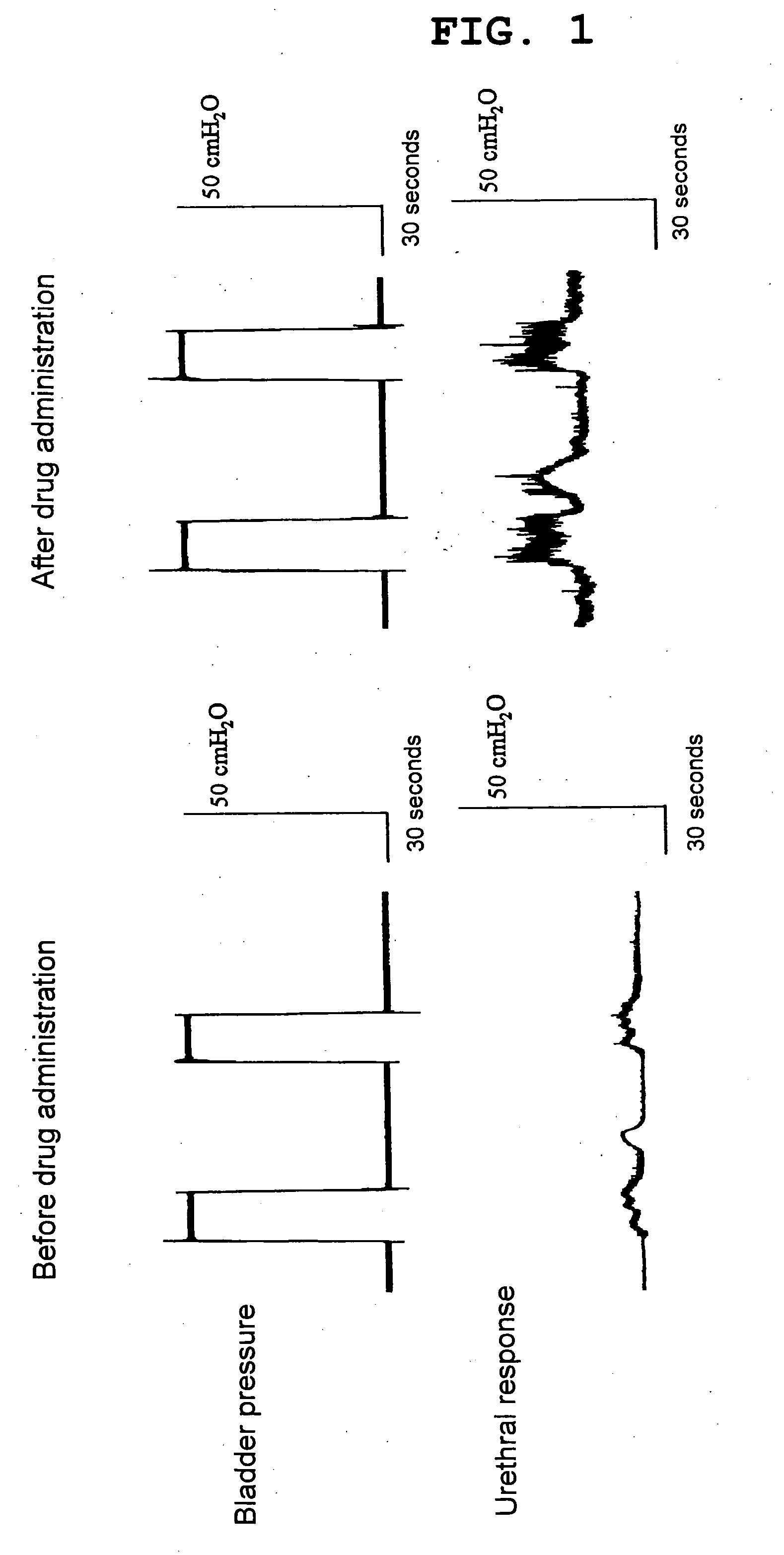
Methods for treating lower urinary tract disorders using alpha2delta subunit calcium channel modulators with smooth muscle modulators
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g. gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:EDUSA PHARMA
Methods for decreasing detrusor muscle overactivity
InactiveUS20050239890A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsDiseaseGabapentin
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs, e.g., gabapentin and pregabalin, fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Non-invasive electrical and magnetic nerve stimulators used to treat overactive bladder and urinary incontinence
Transcutaneous electrical and magnetic nerve stimulation devices and methods generate energy that is delivered noninvasively to selected nerve fibers within the patient to treat lower urinary tract disorders. The disorders comprise overactive bladder, urge incontinence, stress, incontinence, urge frequency, non-obstructive urinary retention and interstitial cystitis / painful bladder syndrome. In preferred embodiments, a posterior tibial nerve of a patient is stimulated non-invasively to treat bladder disorders.
Owner:ELECTROCORE
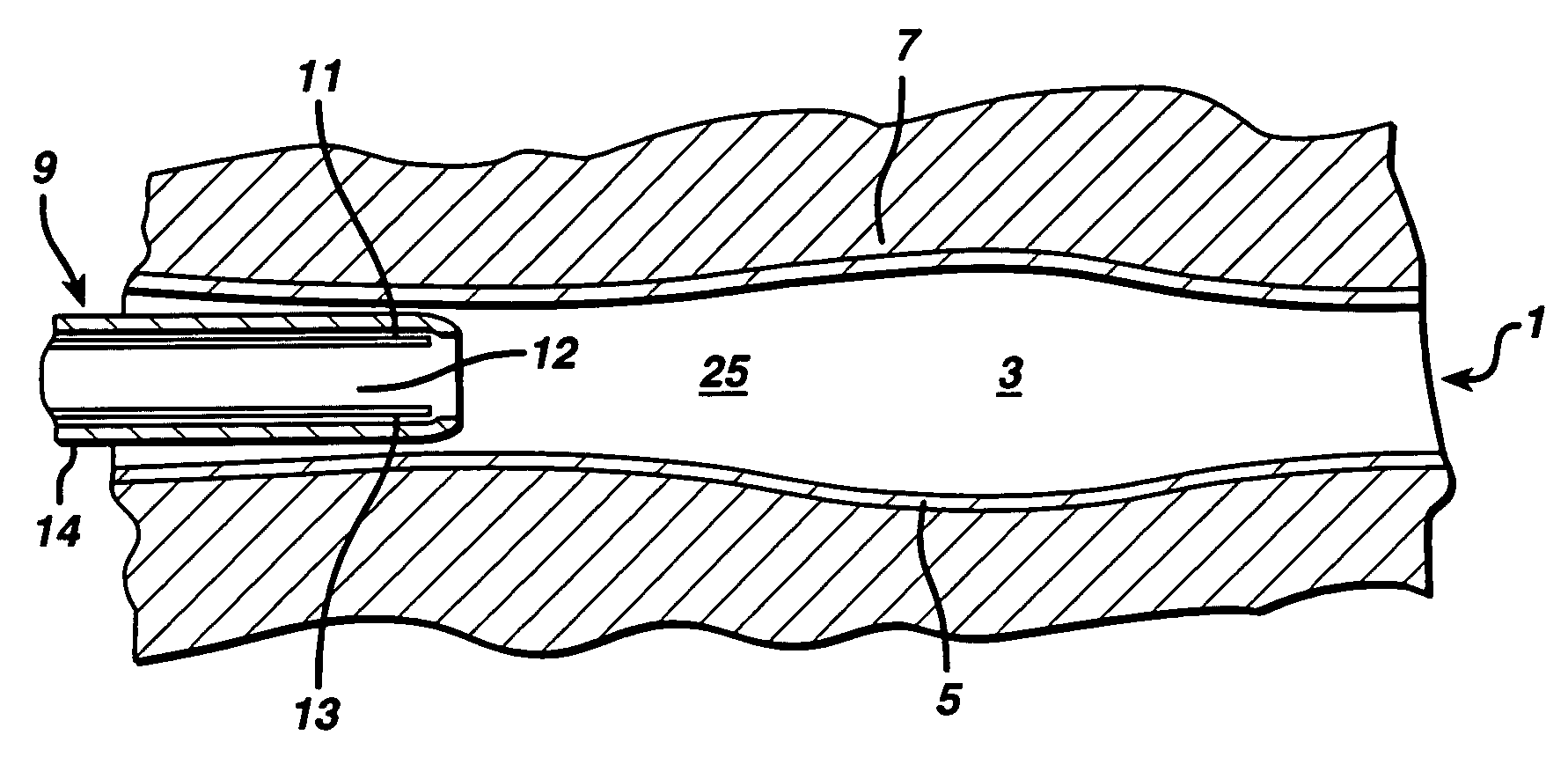
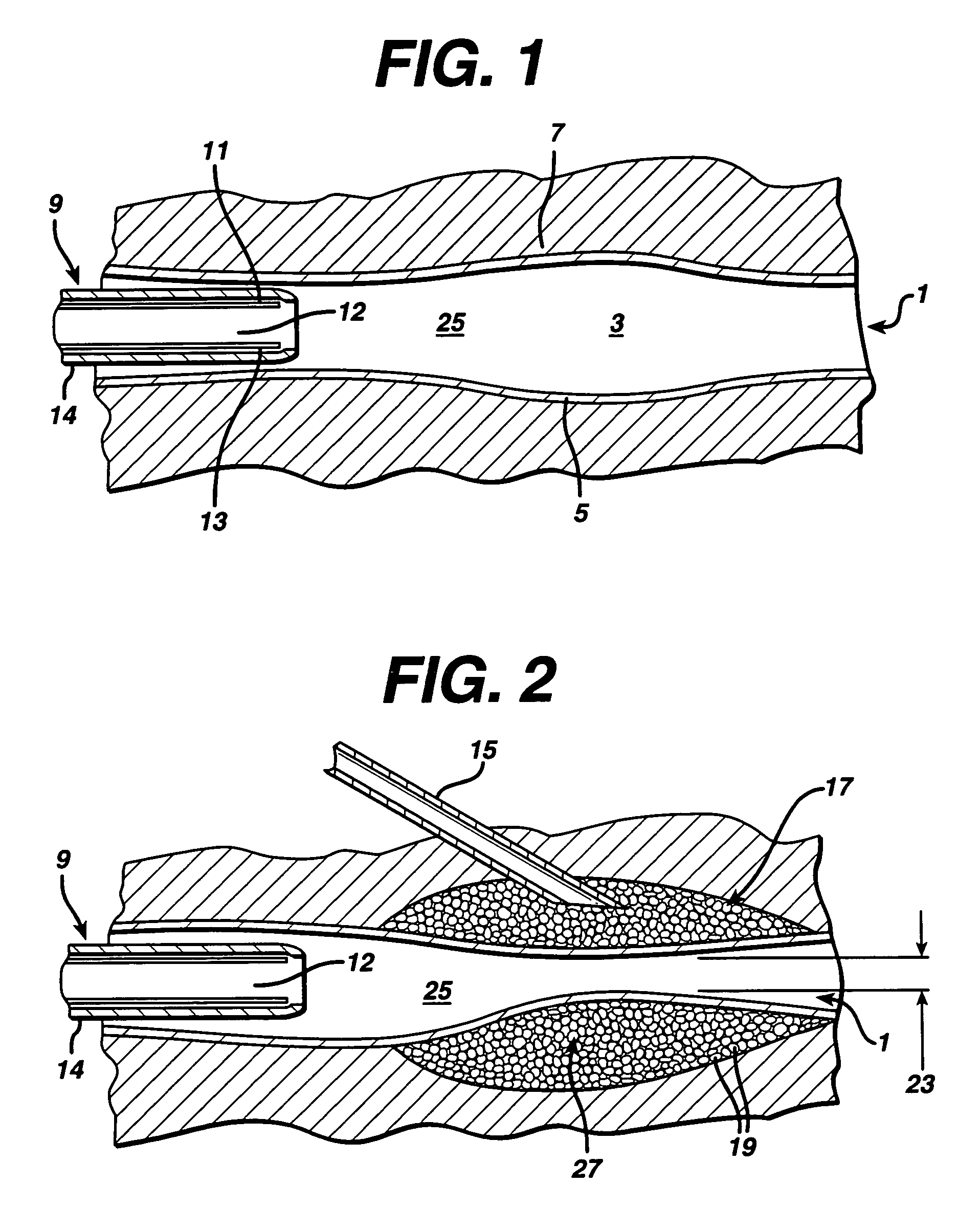
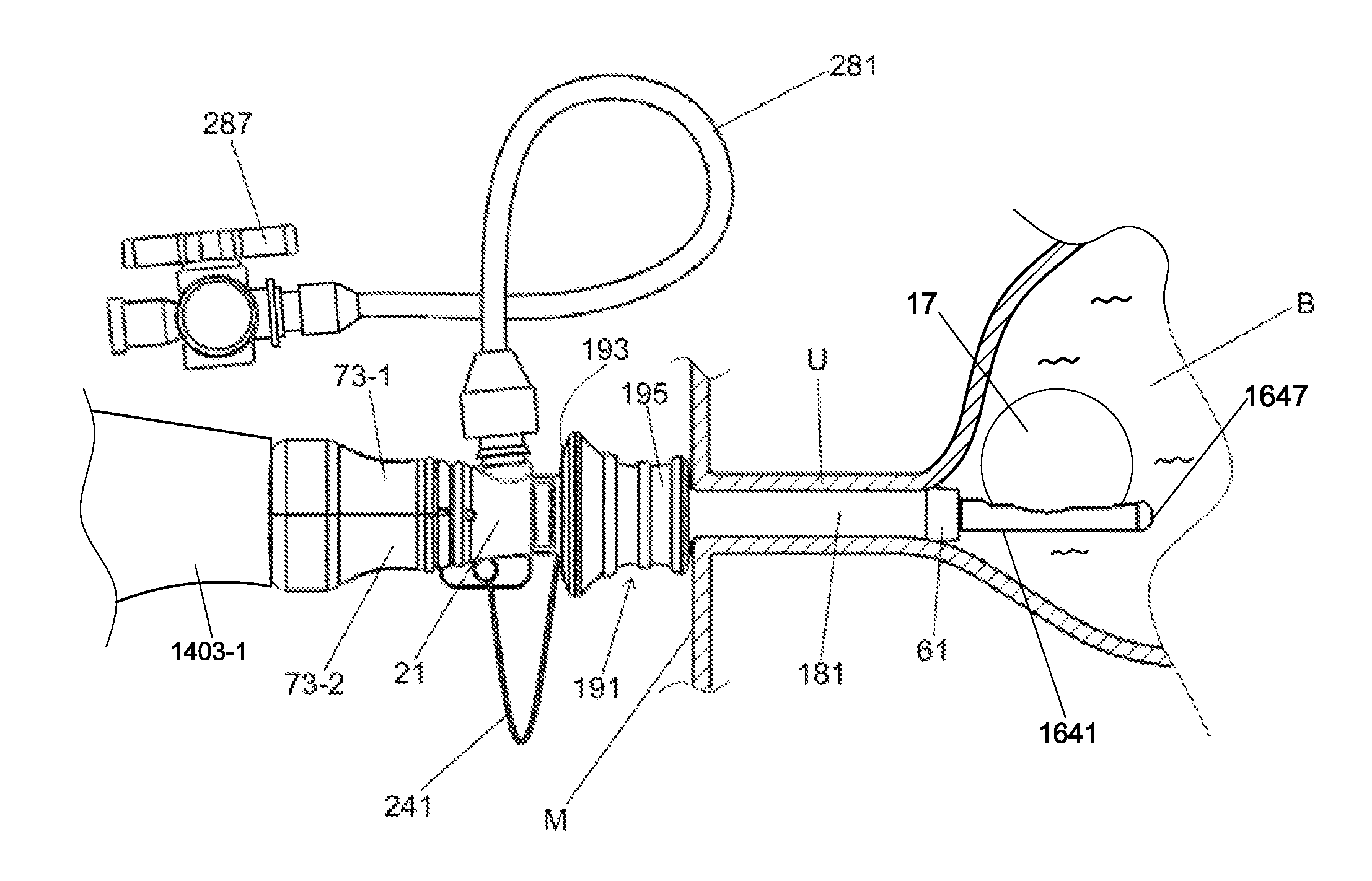
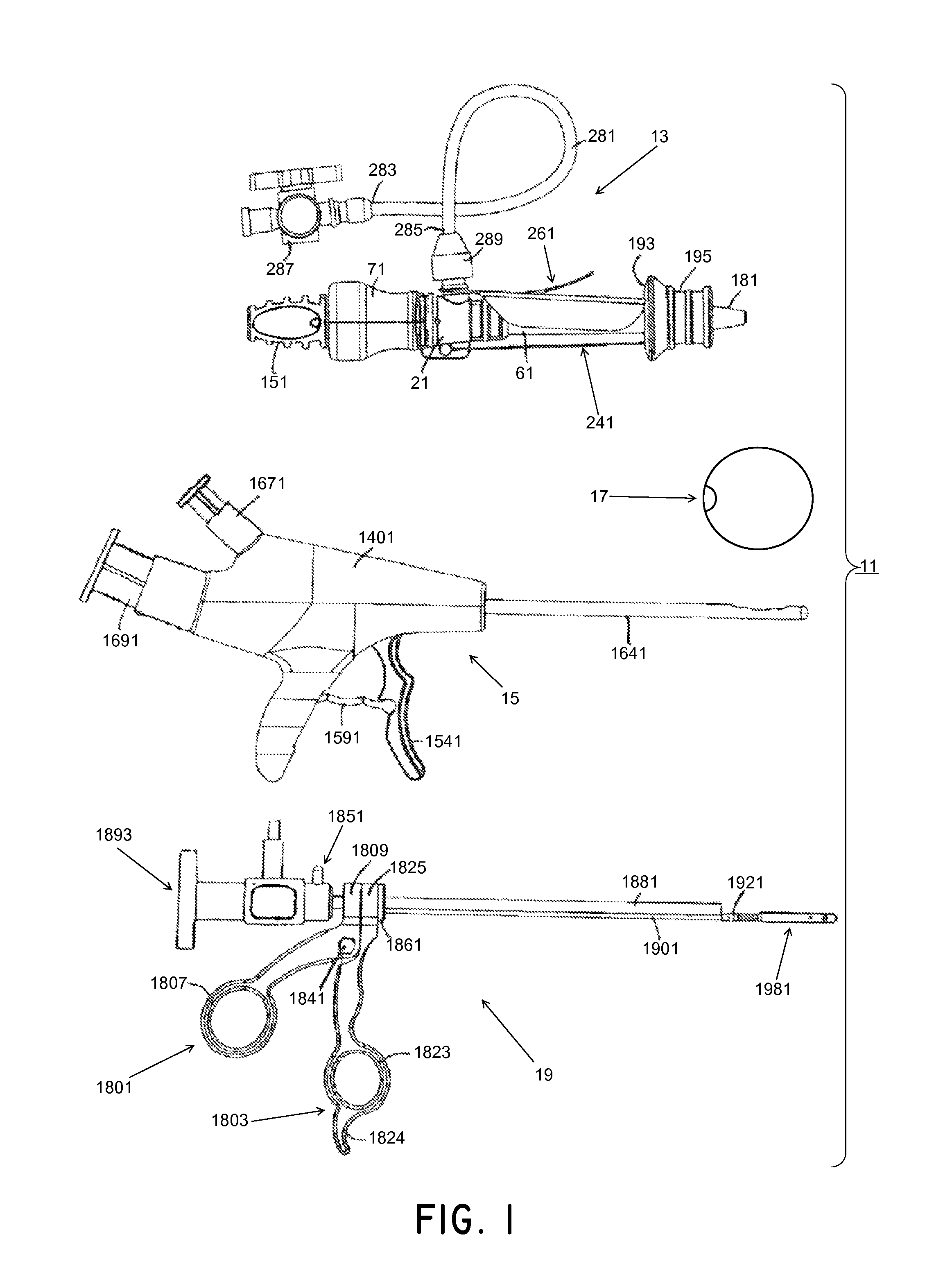
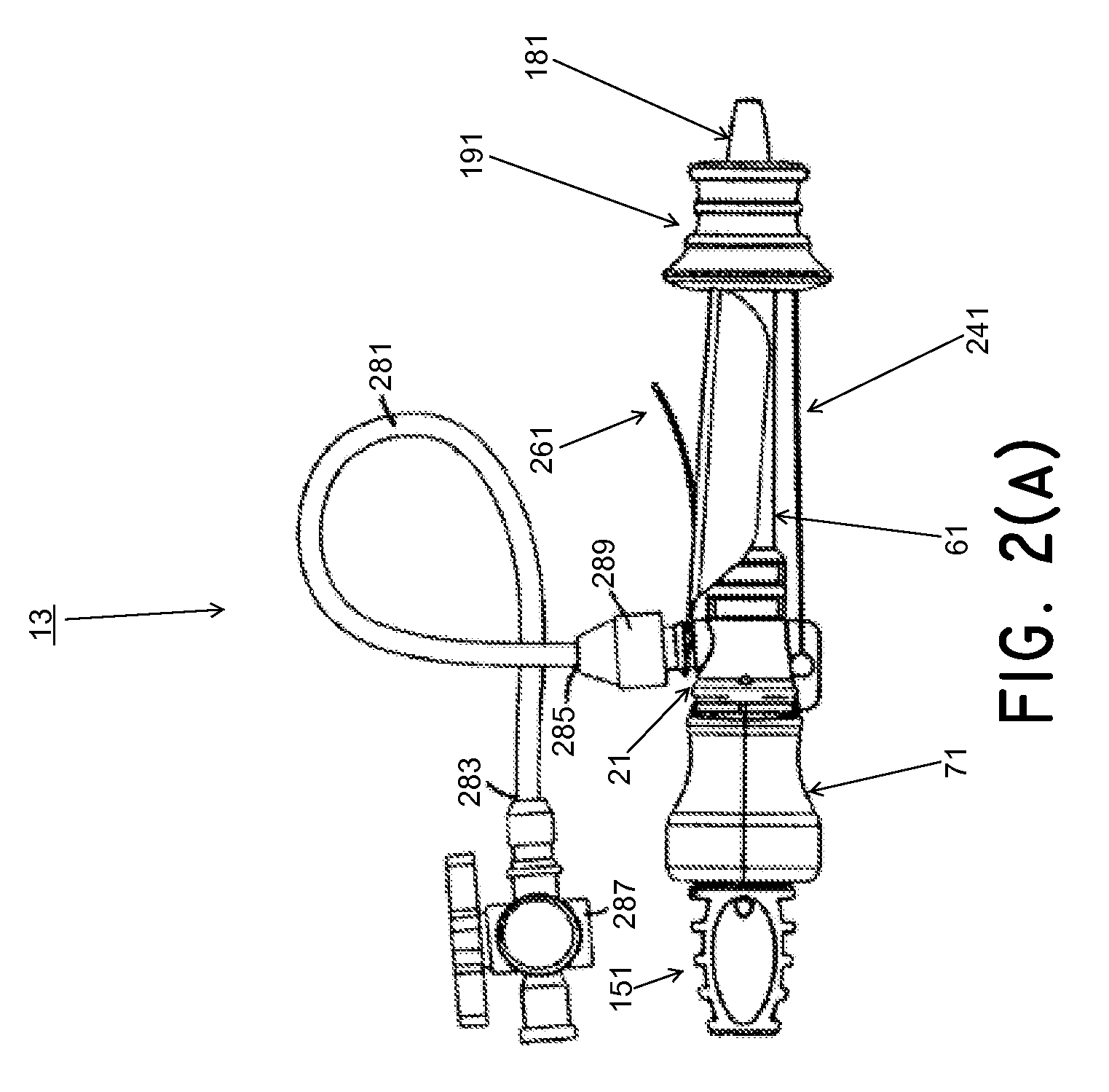
Methods and systems for performing a medical procedure
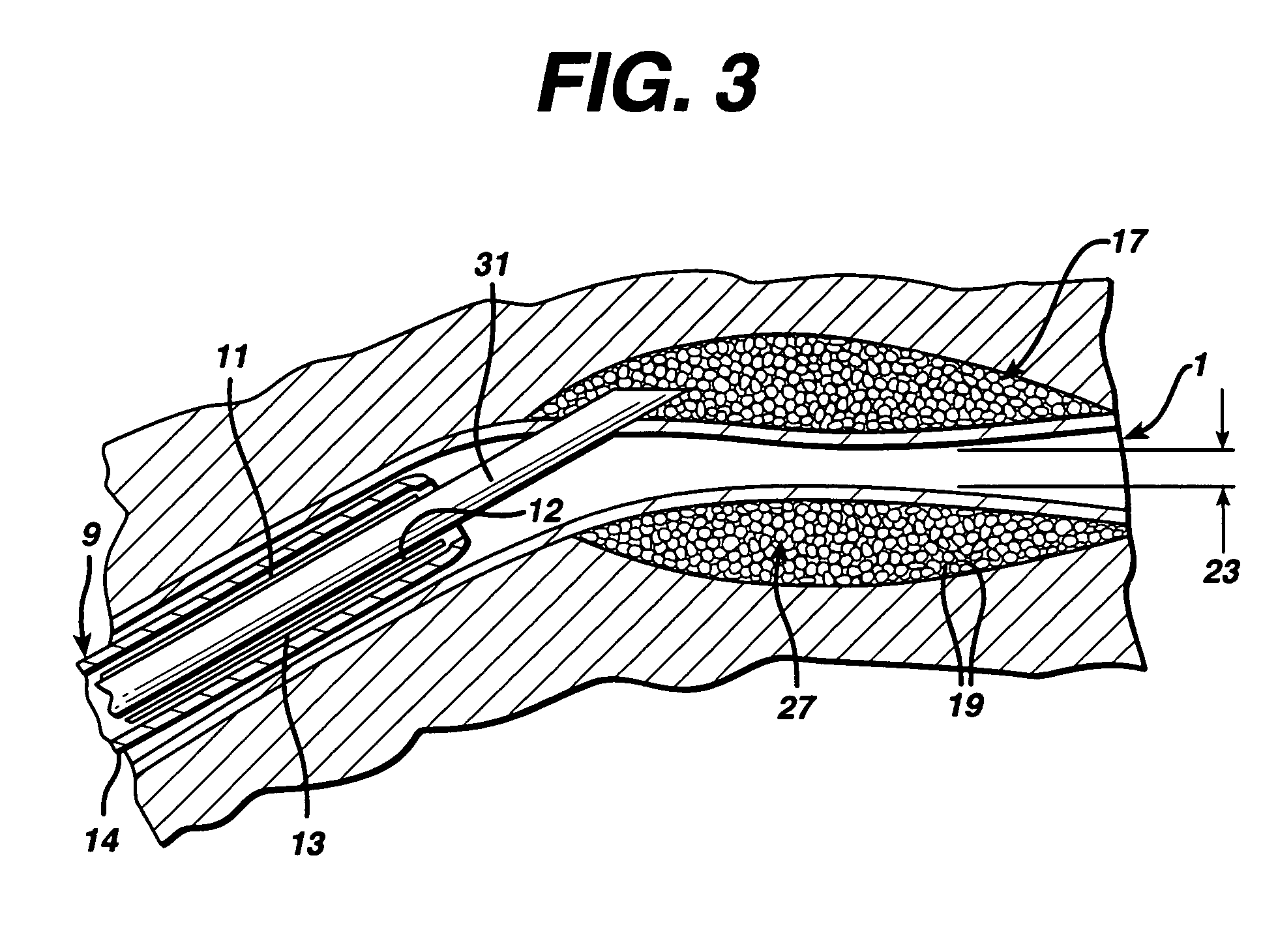
Method and system for treating a patient using a compressible, pressure-attenuating device. According to one embodiment, the system is used to treat urinary tract disorders and comprises an access device, a delivery device, a pressure-attenuating device, and a removal device. The access device may be used to create a passageway to an anatomical structure, such as the patient's bladder. The delivery device may be inserted through the passageway created by the access device and may be used to deliver the pressure-attenuating device to the anatomical structure. The removal device may be inserted through the passageway created by the access device and may be used to view the bladder and / or to capture, to deflate and to remove the pressure-attenuating device.
Owner:SOLACE THERAPEUTICS
Methods for treating lower urinary tract disorders using alpha2delta subunit calcium channel modulators with smooth muscle modulators
InactiveUS20060247311A1Need lessLimited efficacyBiocidePeptide/protein ingredientsDiseaseGabapentinoid
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g. gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
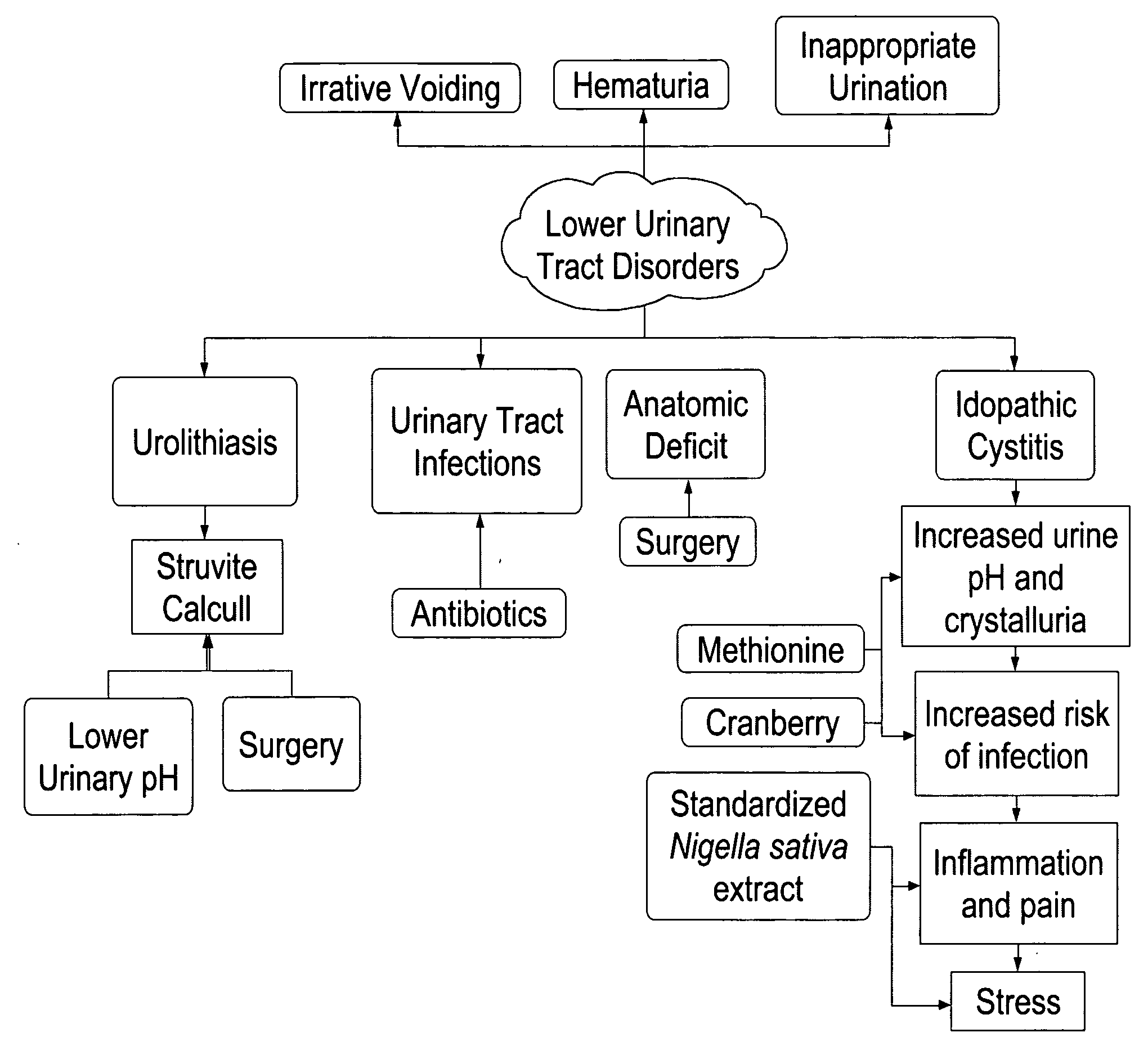
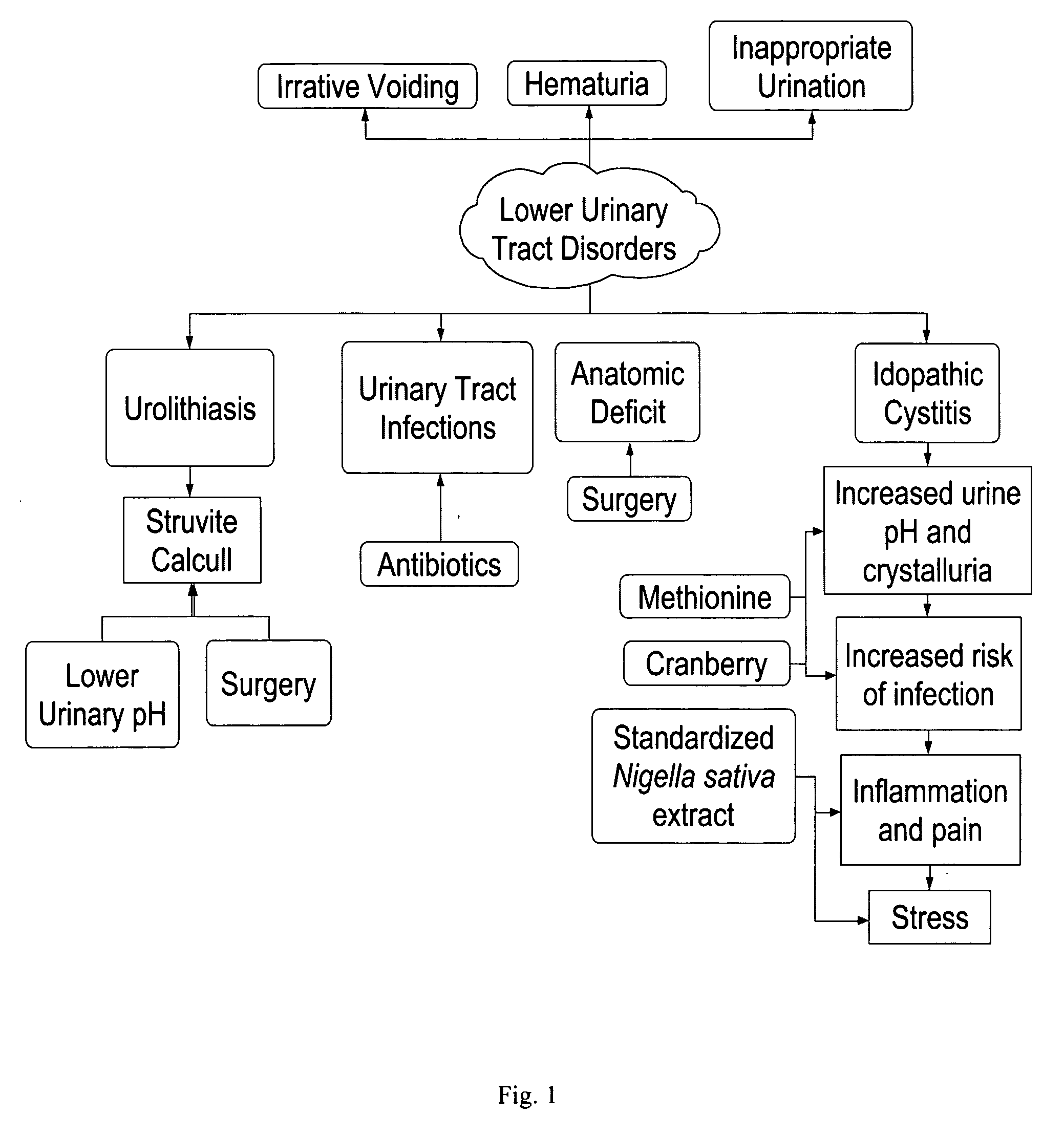
Formulations containing thymoquinone for urinary health
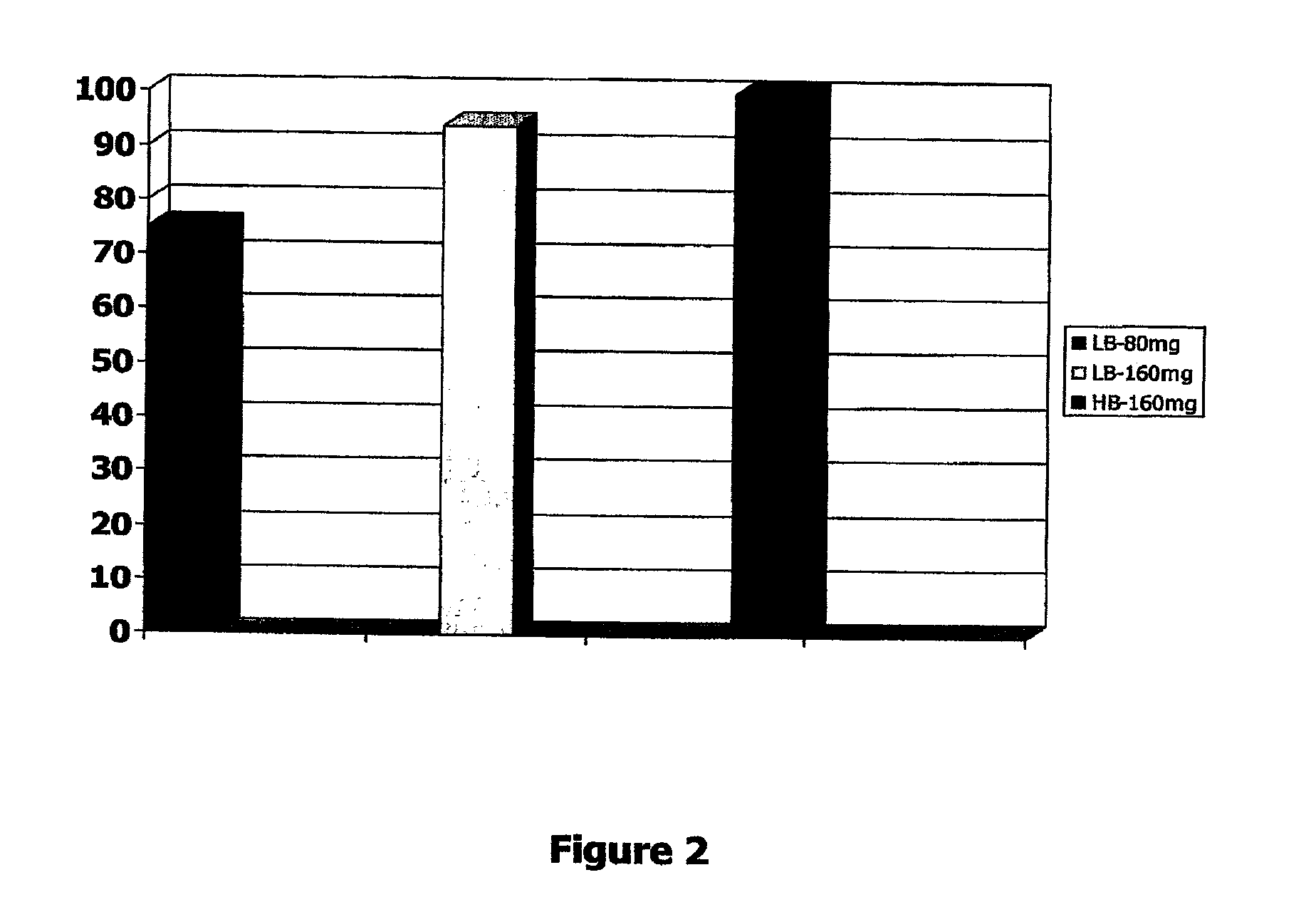
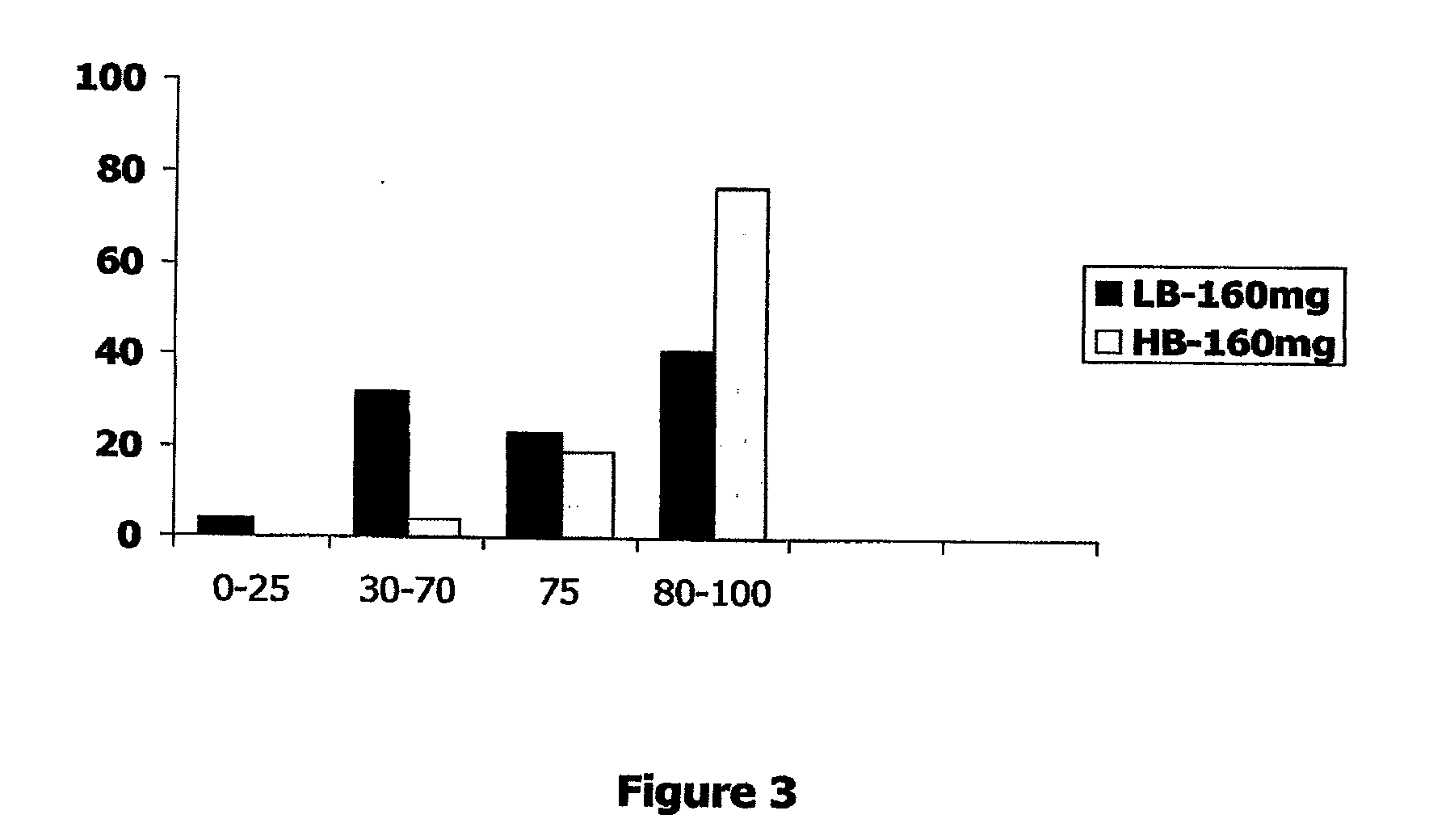
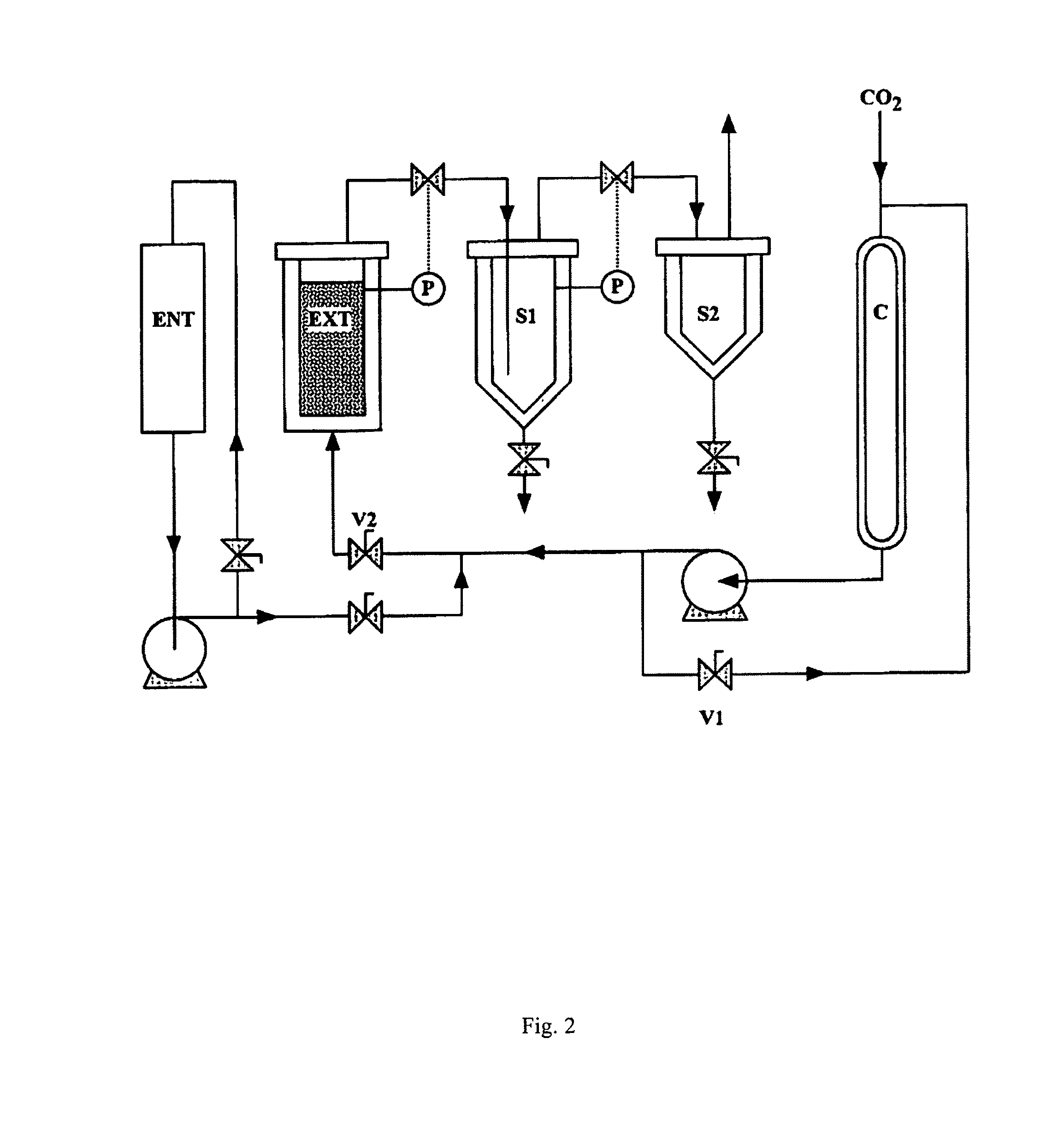
InactiveUS20100028468A1Reduced pHReduce physical stressOrganic active ingredientsBiocideDiseaseCRANBERRY JUICE
A novel dietary supplement or therapeutic composition is provided that serves to treat lower urinary tract disorders or to support normal urinary tract function in animals. The supplement comprises a thymoquinone formulation and at least one member of the group consisting of cranberry fruit, cranberry extract, cranberry juice and a pharmaceutical grade methionine.
Owner:POPULATION RES
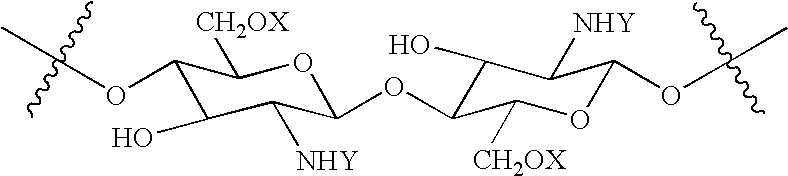
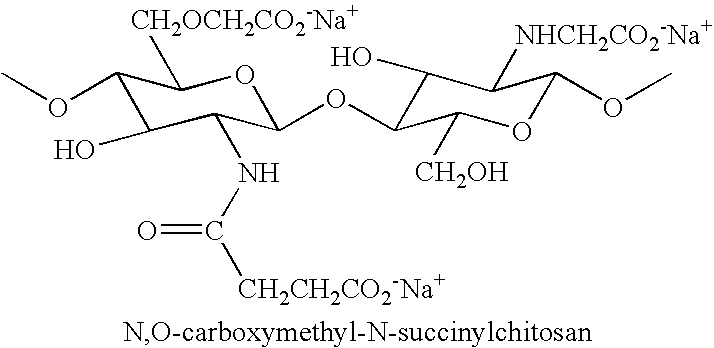
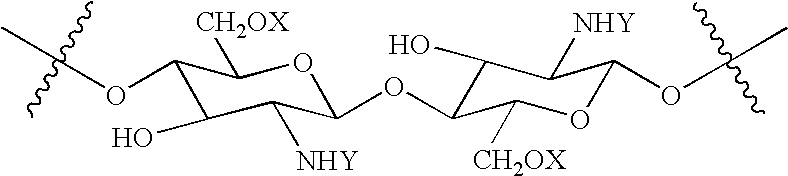
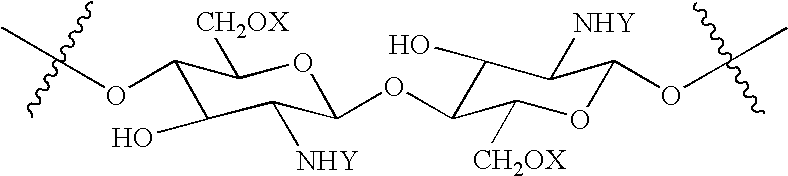
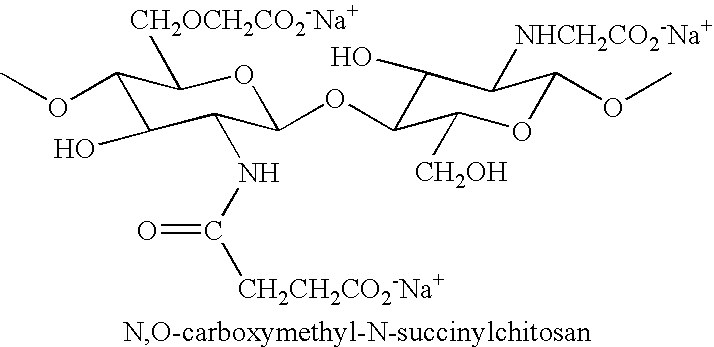
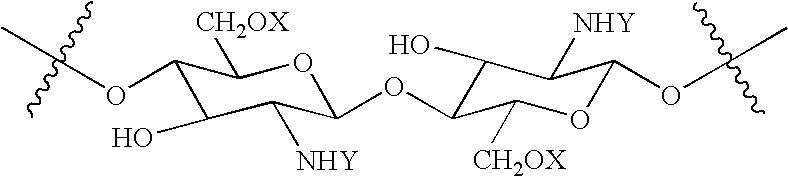
N-acylated chitinous polymers and methods of use thereof
InactiveUS20050214255A1Expanded solubility rangeOrganic active ingredientsBiocideDiseaseNervous system
The invention pertains to N-acetylated, N, O-carboxyalkylchitosans and methods for using the chitosans to treat disorders, such as cancer, nervous system disorders, urinary tract disorders, and reproductive tract disorders.
Owner:CHITOGENICS
Kits and improved compositions for treating lower urinary tract disorders
PendingUS20120196830A1Remarkable effectSufficient quantityAntibacterial agentsBiocideDiseaseSevere pain
Superior buffered formulations and their kits for treating lower urinary tract symptoms and disorders are provided in the invention. In particular superior buffered formulations have demonstrated improvement for treating lower urinary tract symptoms of patients experiencing severe pain and / or urgency of the bladder and associated areas of the lower urinary tract.
Owner:PLATINUM MONTAUR LIFE SCI LLC & AS AGENT
Formulations containing thymoquinone for urinary health
InactiveUS8029831B2Reduced pHReduce frequencyBiocideOrganic active ingredientsCRANBERRY JUICEDietary supplement
A novel dietary supplement or therapeutic composition is provided that serves to treat lower urinary tract disorders or to support normal urinary tract function in animals. The supplement comprises a thymoquinone formulation and at least one member of the group consisting of cranberry fruit, cranberry extract, cranberry juice and a pharmaceutical grade methionine.
Owner:POPULATION RES
Method of treating lower urinary tract disorders
The invention relates to a method of treating at least one symptom of a lower urinary tract disorder in a subject in need of treatment wherein the symptom is selected from the group consisting of urinary frequency, urinary urgency, urinary urge incontinence, nocturia and enuresis. The method comprises administering to a subject in need of treatment a therapeutically effective amount of a compound that has 5-HT3 receptor antagonist activity and NorAdrenaline Reuptake Inhibitor (NARI) activity. The invention further relates to a method of treating at least one symptom of a lower urinary tract disorder in a subject in need of treatment wherein the symptom is selected from the group consisting of urinary frequency, urinary urgency, urinary urge incontinence, nocturia and enuresis, comprising coadministering to said subject a first amount of a 5-HT3 antagonist and a second amount of a NARI, wherein the first and second amounts together comprise a therapeutically effective amount or are each present in a therapeutically effective amount.
Owner:DYNOGEN PHARM INC
Methods for treating lower urinary tract disorders and the related disorders vulvodynia and vulvar vestibulitis using Cav2.2 subunit calcium channel modulators
The invention relates to methods of using Cav2.2 subunit calcium channel modulators to treat painful and non-painful lower urinary tract disorders and the related genitourinary tract disorders vulvodynia and vulvar vestibulitis in normal and spinal cord injured patients.
Owner:EDUSA PHARMA
Methods and systems for performing a medical procedure
Method and system for treating a patient using a compressible, pressure-attenuating device. According to one embodiment, the system is used to treat urinary tract disorders and can include one or more of an access device, a delivery device, a pressure-attenuating device, and a removal device. The access device may be used to create a passageway to an anatomical structure, such as the patient's bladder. The delivery device may be inserted through the passageway created by the access device and may be used to deliver the pressure-attenuating device to the anatomical structure. The removal device may be inserted through the passageway created by the access device and may be used to view the bladder and / or to capture, to deflate and to remove the pressure-attenuating device.
Owner:SOLACE THERAPEUTICS
N-acylated chitinous polymers and methods of use thereof
The invention pertains to N-acetylated, N, O-carboxyalkylchitosans and methods for using the chitosans to treat disorders, such as cancer, nervous system disorders, urinary tract disorders, and reproductive tract disorders.
Owner:CHITOGENICS
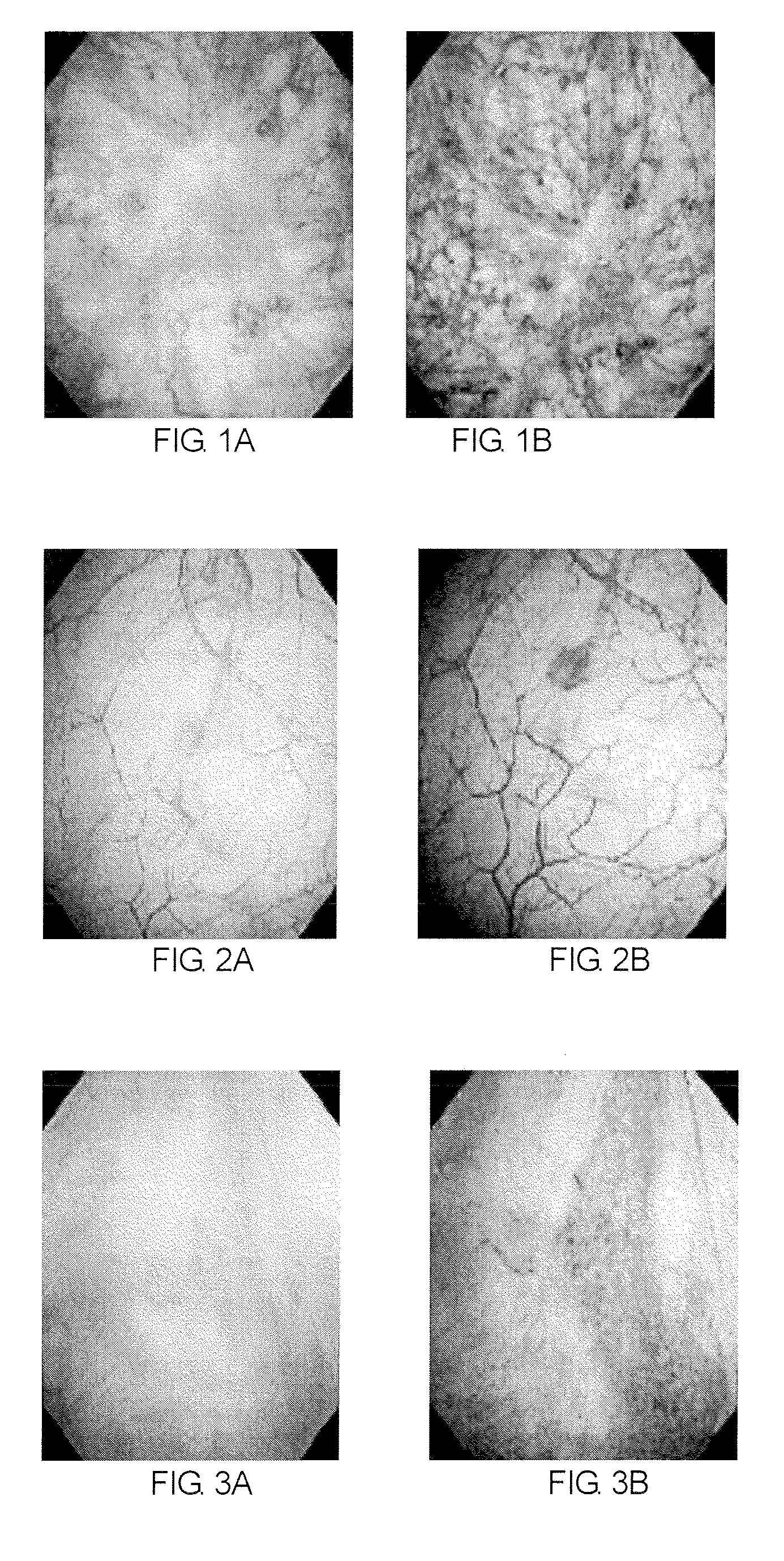
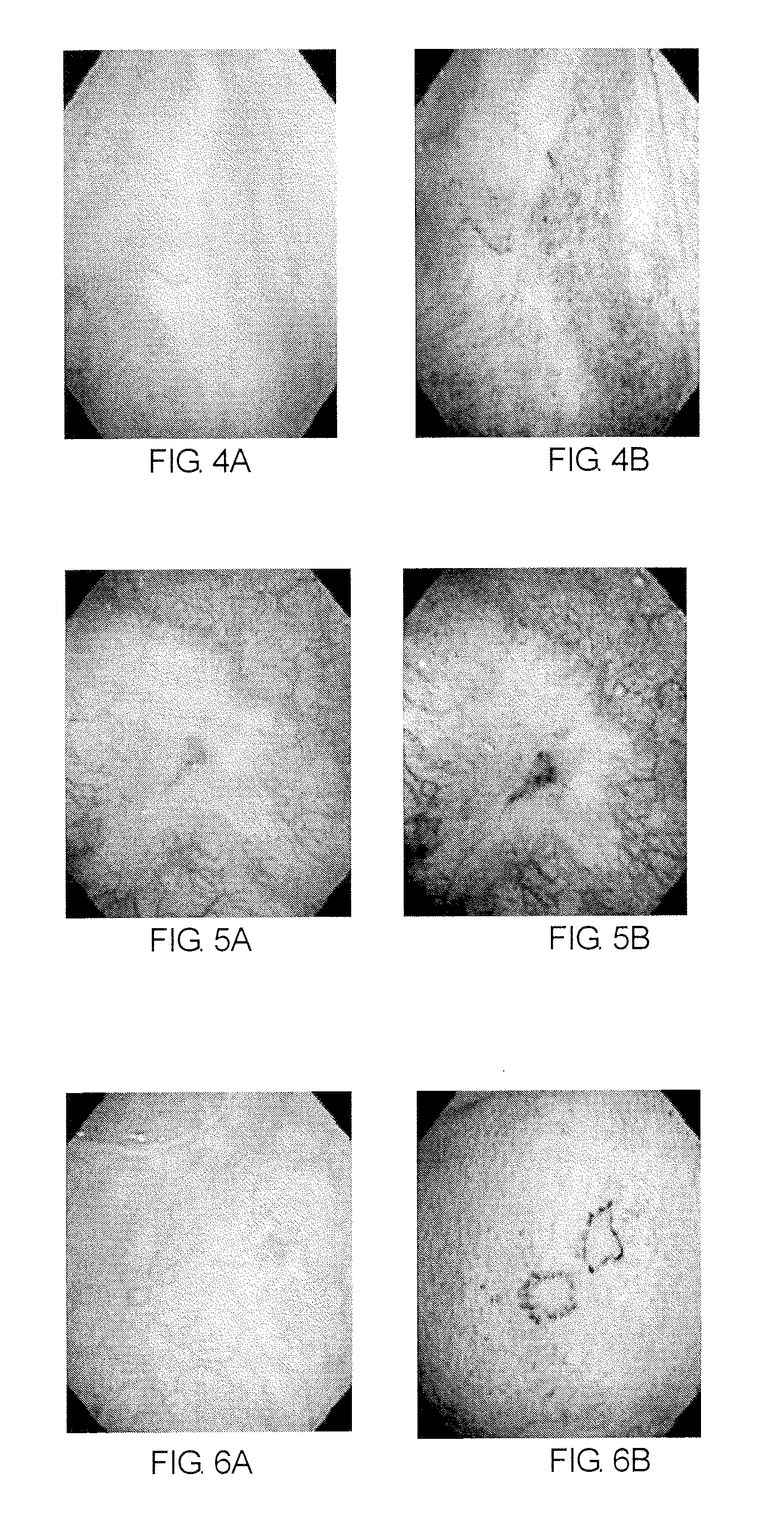
Method of diagnosing a lower urinary tract disorder
A method for sensitively diagnosing lower urinary tract disorders, particularly interstitial cystitis, is provided. The method can visually, simply, and clearly diagnose bladder ulcer without requirement of bladder hydrodistention under anesthesia. In the method, lower urinary tract disorders are diagnosed by observing an abnormality of a blood vessel and / or a newly formed blood vessel in a surface of bladder mucous membrane among abnormalities in blood vessels and / or newly formed blood vessels by visually comparing an image of the surface of the bladder mucous membrane and an image of a deep portion of the bladder mucous membrane obtained using a bladder endoscope system having a narrow band imaging device.
Owner:N M A
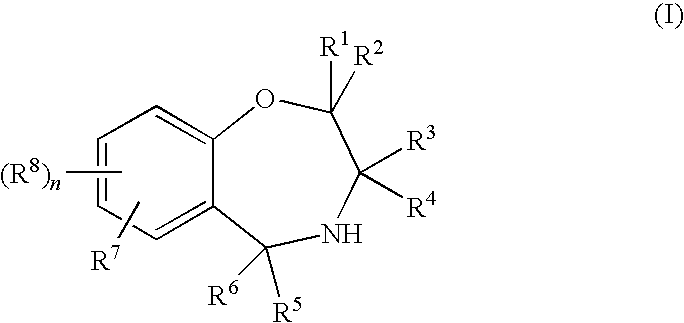
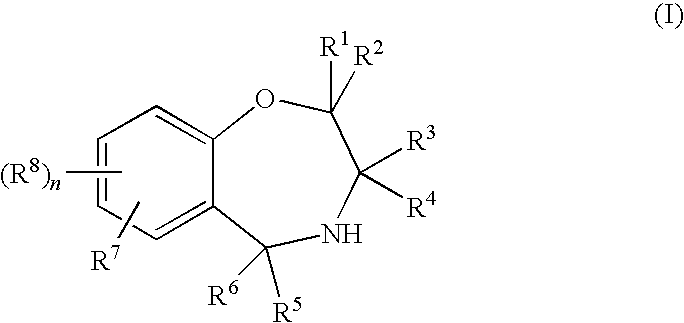
Benzoxazepine derivatives and use thereof
InactiveUS20100087418A1Superior serotonin -HT2C receptor activation actionLow toxicityBiocideSenses disorderSerotoninMedicine
Compounds represented by the general formula (I):wherein each symbol is as defined in the description [with the proviso that 9-chloro-7-(1,1-dimethylethyl)-2,3,4,5-tetrahydro-1,4-benz-oxazepine and N-[[(5S)-2-oxo-3-(2,3,4,5-tetrahydro-1,4-benz-oxazepin-7-yl)-5-oxazolidinyl]methyl]acetamide are excluded], salts of the same, and prodrugs thereof have selective activation effect on serotonin 5-HT2C receptor and are useful as preventive and therapeutic agents for lower urinary tract diseases, obesity, and / or pelvic organ prolapse.
Owner:TAKEDA PHARMA CO LTD
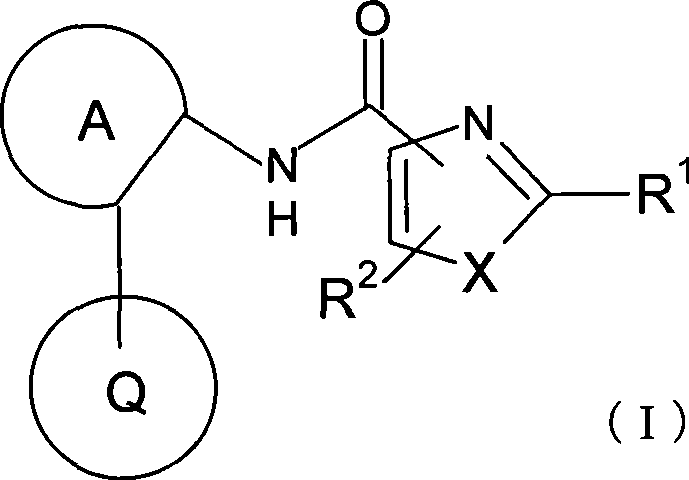
Azolecarboxamide derivative
InactiveCN101426774APotent trkA receptor inhibitionNervous disorderOrganic chemistryDiseaseInterstitial cystitis
To provide a therapeutic or prophylactic agent for frequent urination / strong urge to urinate or incontinence associated with overactive bladder, a lower urinary tract disease accompanied by a lower urinary tract pain such as interstitial cystitis and chronic prostatitis or a disease accompanied by a pain, which acts based on excellent trkA receptor-inhibiting activity. [MEANS FOR SOLVING PROBLEMS] Disclosed is a novel azolecarboxamide derivative having an azole ring (e.g., a thiazole or oxazole ring) bound to a benzene, pyridine or pyrimidine ring through a carboxamide. It is confirmed that the azolecarboxamide derivative has a potent trkA receptor-inhibiting activity. It is found that the azolecarboxamide derivative can be used as a very efficient, safe therapeutic or prophylactic agent for a lower urinary tract disease or a disease accompanied by a pain.
Owner:ASTELLAS PHARMA INC
Methods and systems for performing a medical procedure
Method and system for treating a patient using a compressible, pressure-attenuating device. According to one embodiment, the system is used to treat urinary tract disorders and can include one or more of an access device, a delivery device, a pressure-attenuating device, and a removal device. The access device may be used to create a passageway to an anatomical structure, such as the patient's bladder. The delivery device may be inserted through the passageway created by the access device and may be used to deliver the pressure-attenuating device to the anatomical structure. The removal device may be inserted through the passageway created by the access device and may be used to view the bladder and / or to capture, to deflate and to remove the pressure-attenuating device.
Owner:SOLACE THERAPEUTICS
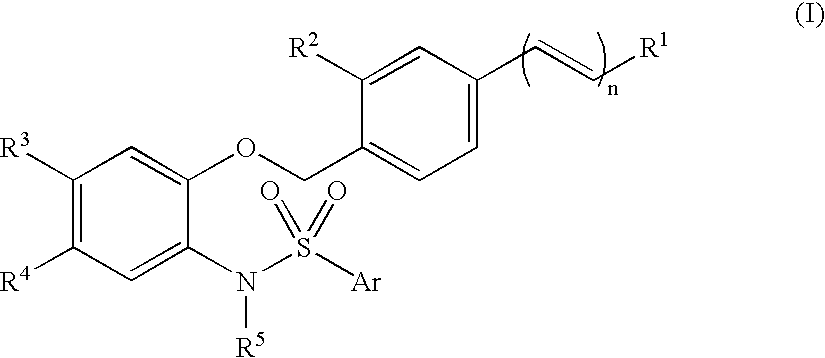
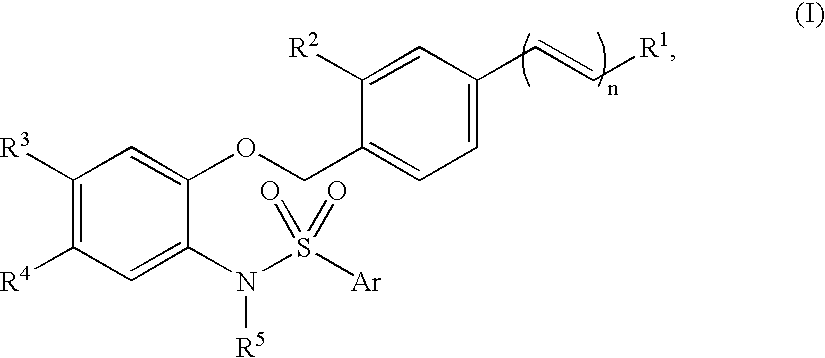
N-phenylarylsulfonamide compound drug containing the compound as active ingredient intermediate for the compound and processes for producing the same
InactiveUS20050124672A1Treatment effectImprove composite effectBiocideNervous disorderProstaglandin bindingIn vivo
An N-phenylarylsulfonylamide compound of formula (I) (R1 is COOH etc.; R2 is hydrogen, methyl, etc.; R3 and R4 are a combination of methyl and methyl, etc.; R5 is isopropyl etc.; Ar is thiazolyl, pyridyl, 5-methyl-2-furyl each optionally substituted with methyl; n is zero or 1), a synthetic intermediate for the compound and a process for its preparation. The compound of formula (I) binds to a prostaglandin E2 receptor, especially an EP1 subtype receptor, and antagonizes it. It is less affected by protein binding, so it has a satisfactory in vivo activity. Therefore, it is considered to be useful as an analgesic, an antipyretic agent, an agent for the treatment of pollakiuria (frequent urination) and / or lower urinary tract disease syndrome or an antineoplastic agent.
Owner:ONO PHARMA CO LTD
Medicament for preventive and/or therapeutic treatment of lower urinary tract symptom
InactiveUS20080207768A1Good prevention effectGood treatment effectBiocidePeptide/protein ingredientsBenzoic acidDisease
A medicament for preventive and / or therapeutic treatment of a lower urinary tract symptom caused by a lower urinary tract disorder, which comprises as an active ingredient a retinoid such as, for example, 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl]benzoic acid
Owner:KEMPHYS
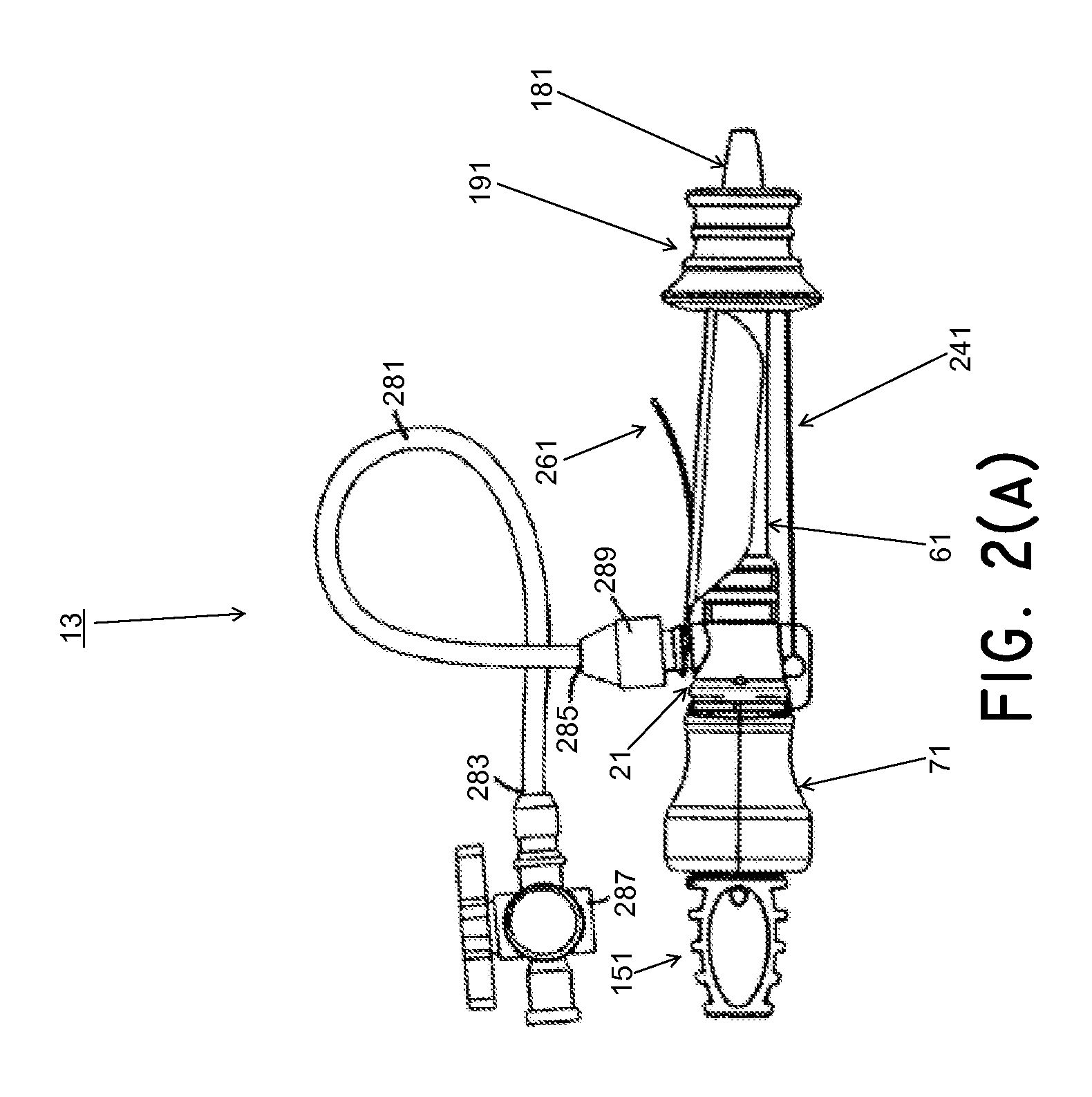
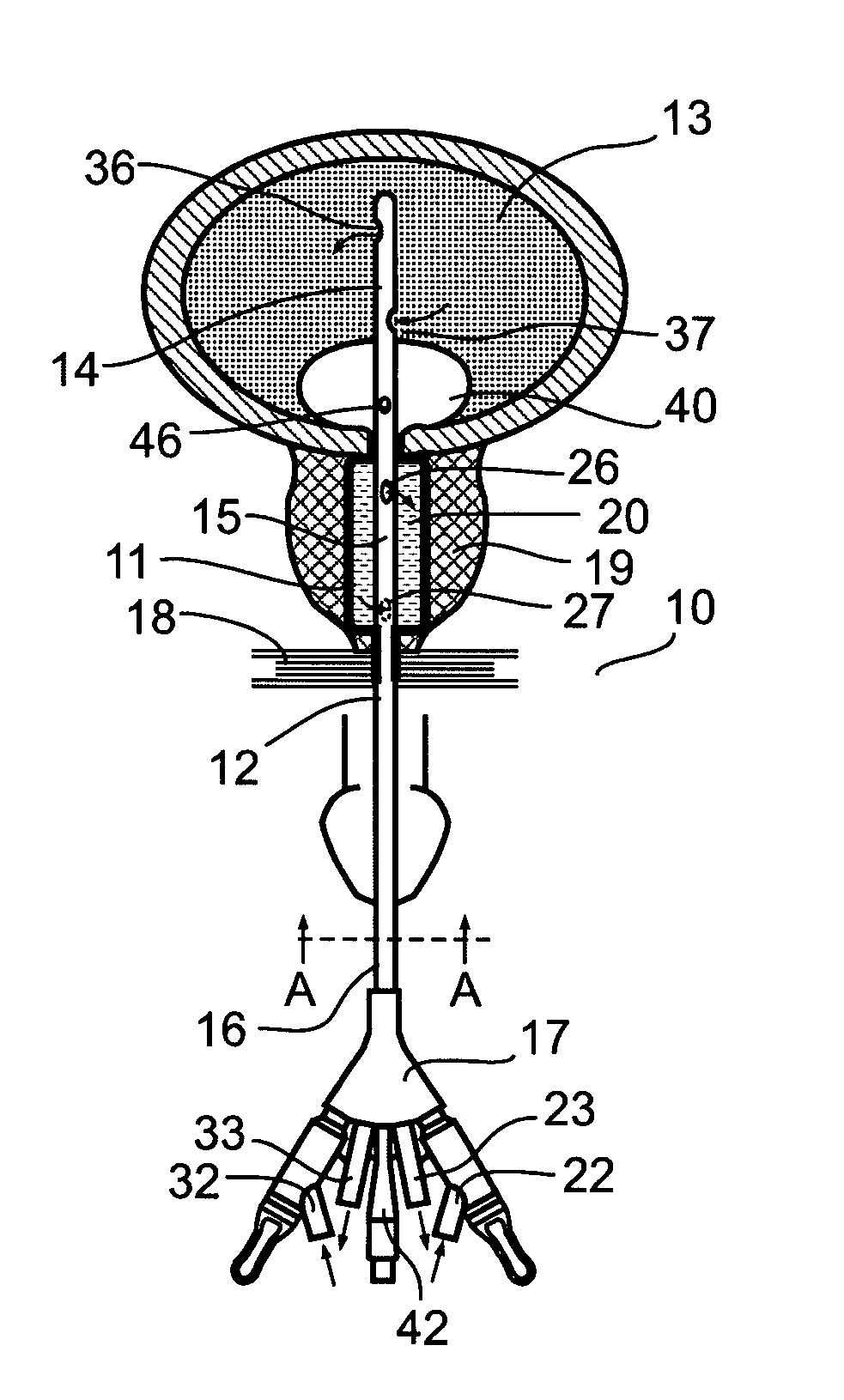
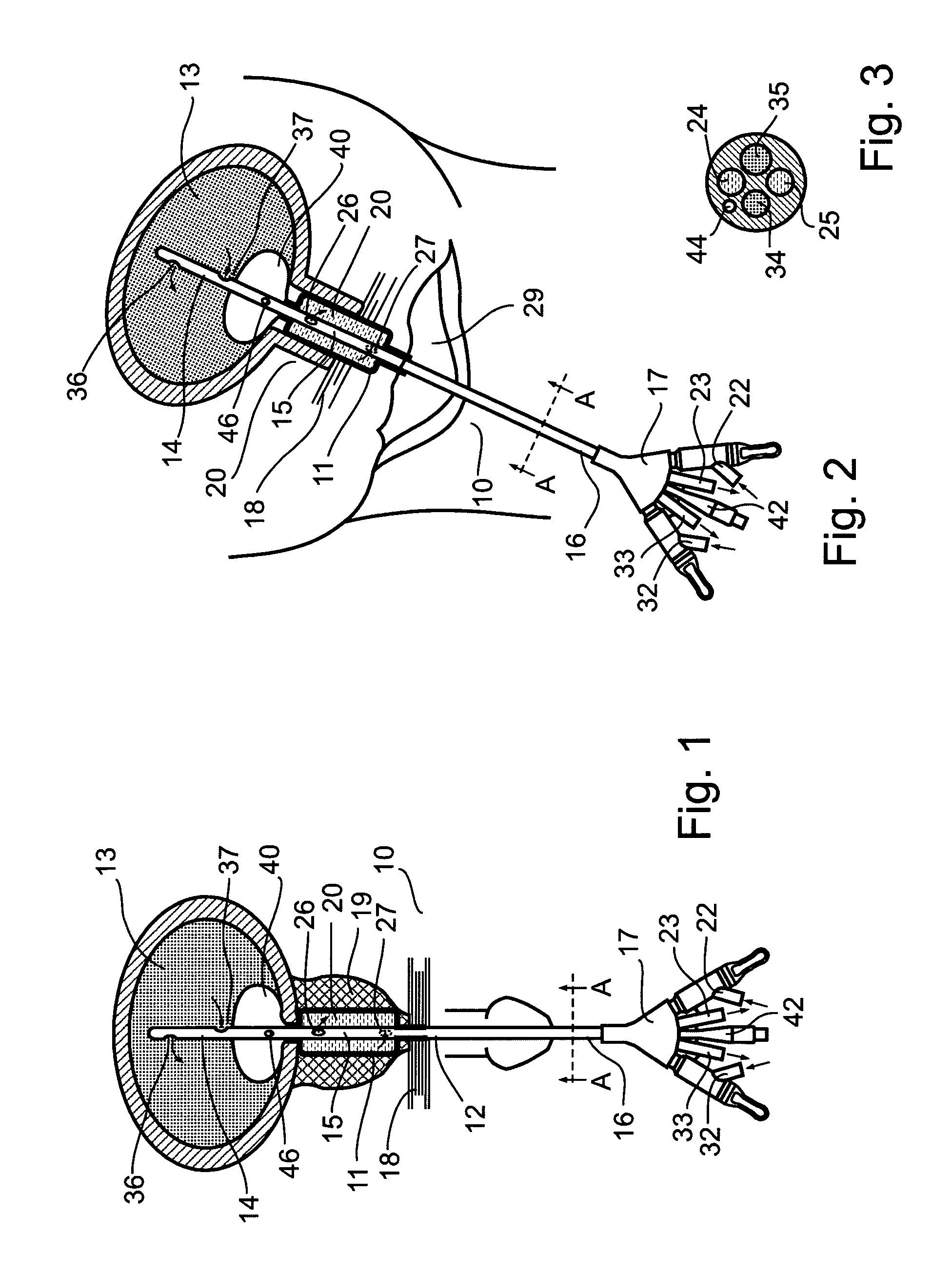
System and method for treating urinary tract disorders
A method, device, and system for treatment of urinary tract disorders is provided. The device includes a catheter having multiple lumens for insertion into a urinary bladder and for providing fluid flow within the bladder and within the fluid reservoir within the urethra. The device further includes an anchor to be placed within the bladder. A port hub having multiple ports is connected at a proximal end of the catheter. A method of treatment includes providing heated fluid to a urinary bladder and to a fluid reservoir for insertion into an adjacent organ such as the urethra. Simultaneous treatment or separate treatment may be done. Optionally, a medicated solution may be added to the heated fluid. Additionally, pressure may be adjusted so as to optimize the therapeutic effect.
Owner:ELMEDICAL
System and method for treating urinary tract disorders
A method, device, and system for treatment of urinary tract disorders is provided. The device includes a catheter having multiple lumens for insertion into a urinary bladder and for providing fluid flow within the bladder and within the fluid reservoir within the urethra. The device further includes an anchor to be placed within the bladder. A port hub having multiple ports is connected at a proximal end of the catheter. A method of treatment includes providing heated fluid to a urinary bladder and to a fluid reservoir for insertion into an adjacent organ such as the urethra. Simultaneous treatment or separate treatment may be done. Optionally, a medicated solution may be added to the heated fluid. Additionally, pressure may be adjusted so as to optimize the therapeutic effect.
Owner:ELMEDICAL
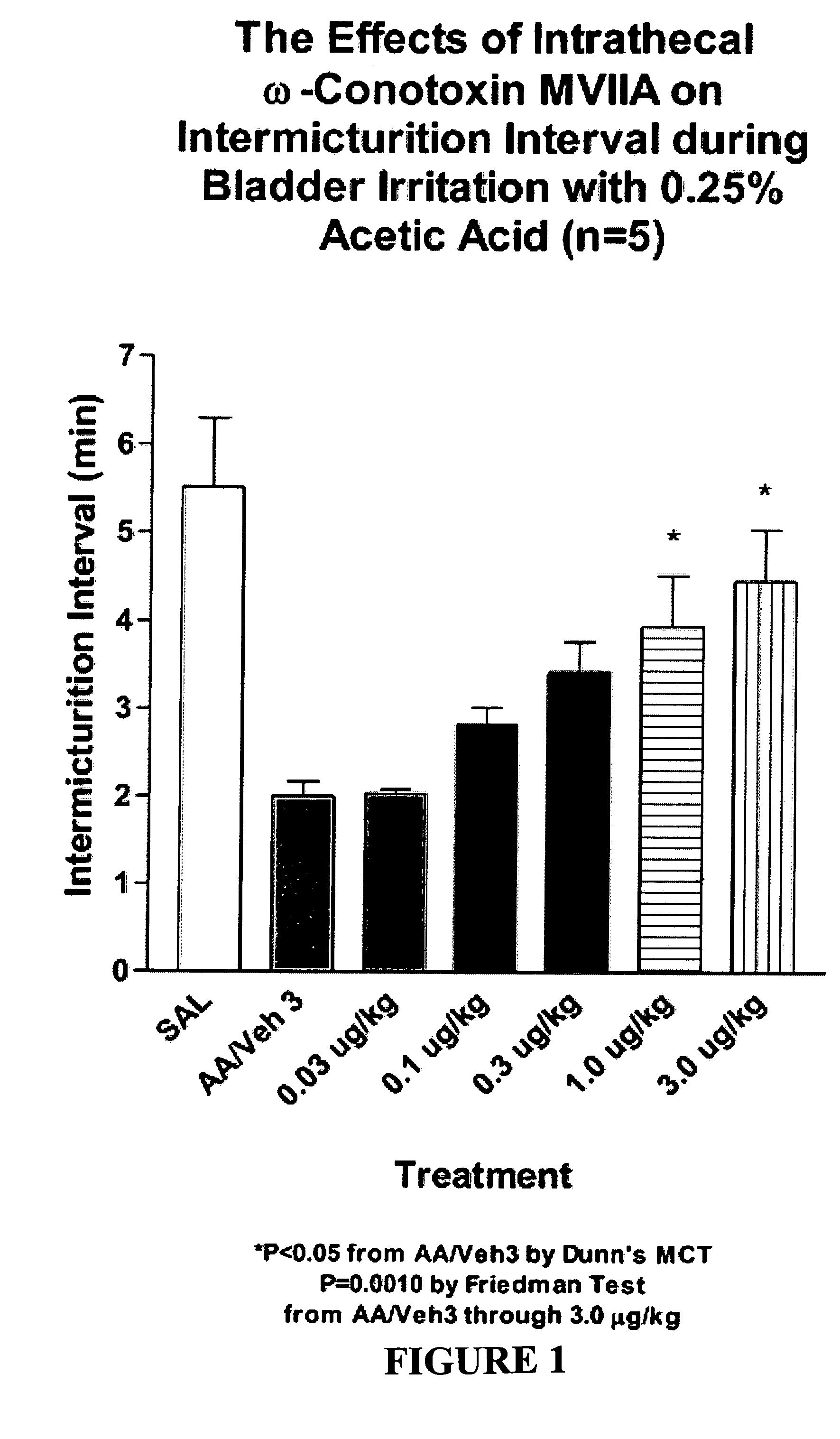
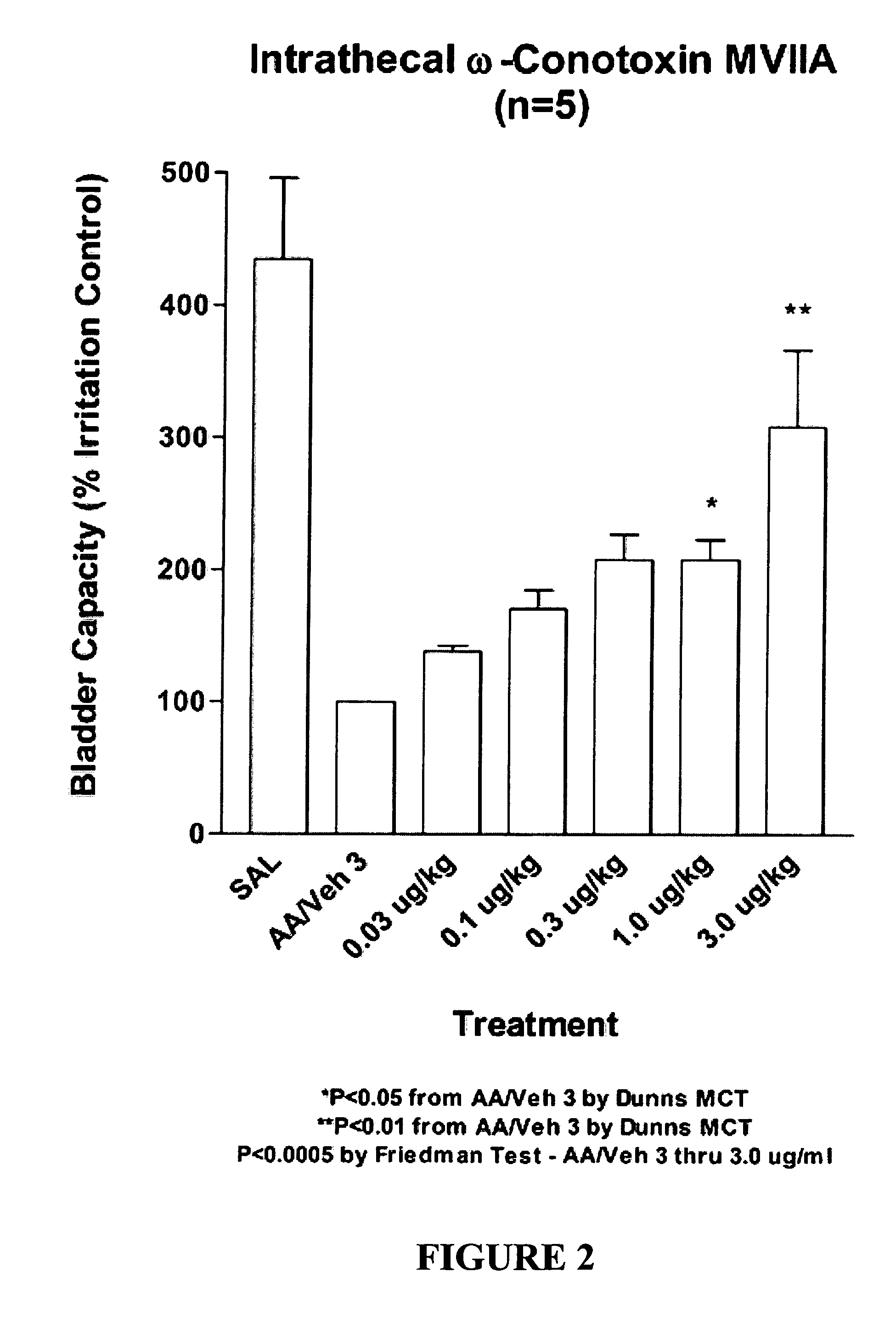
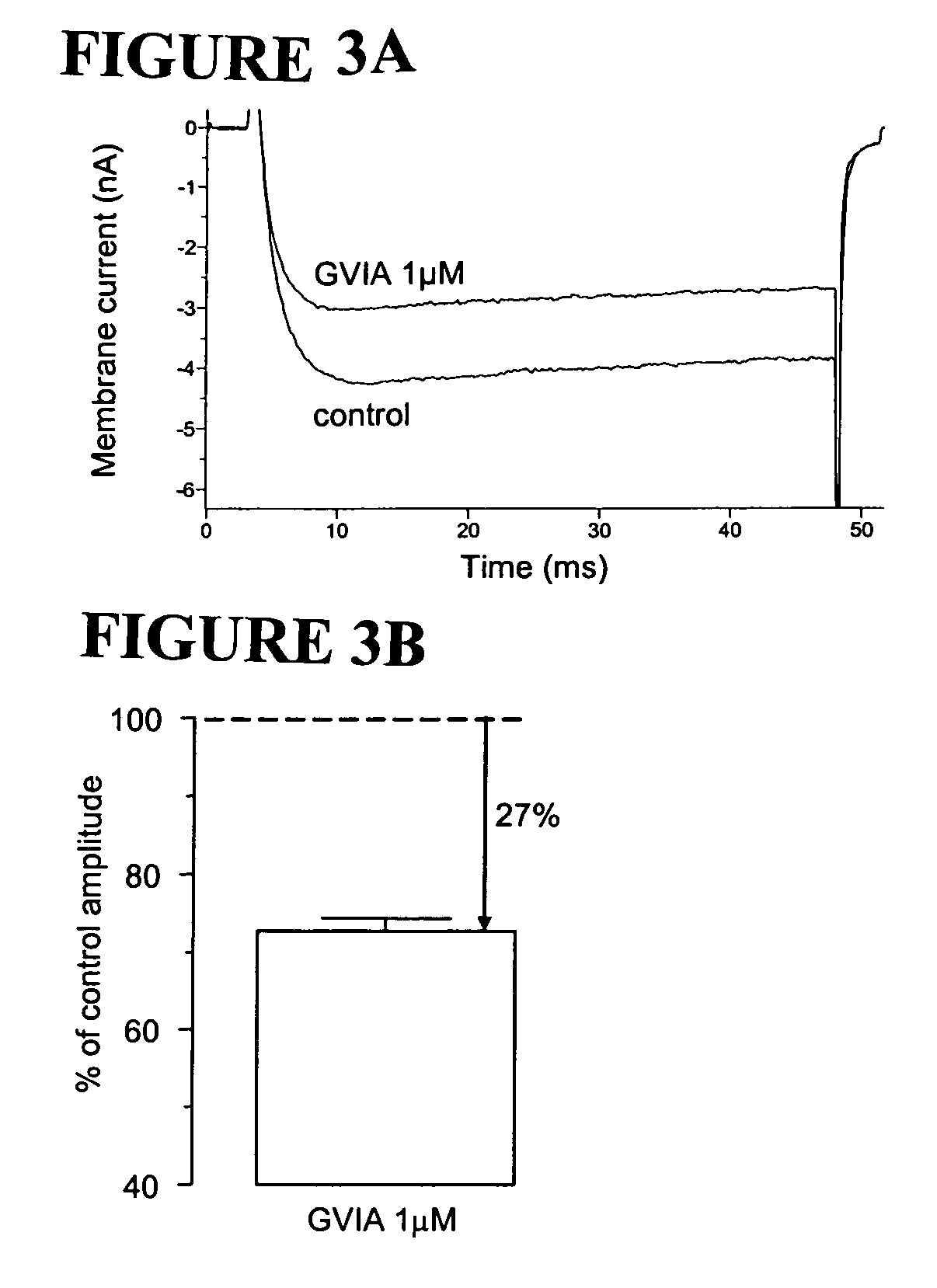
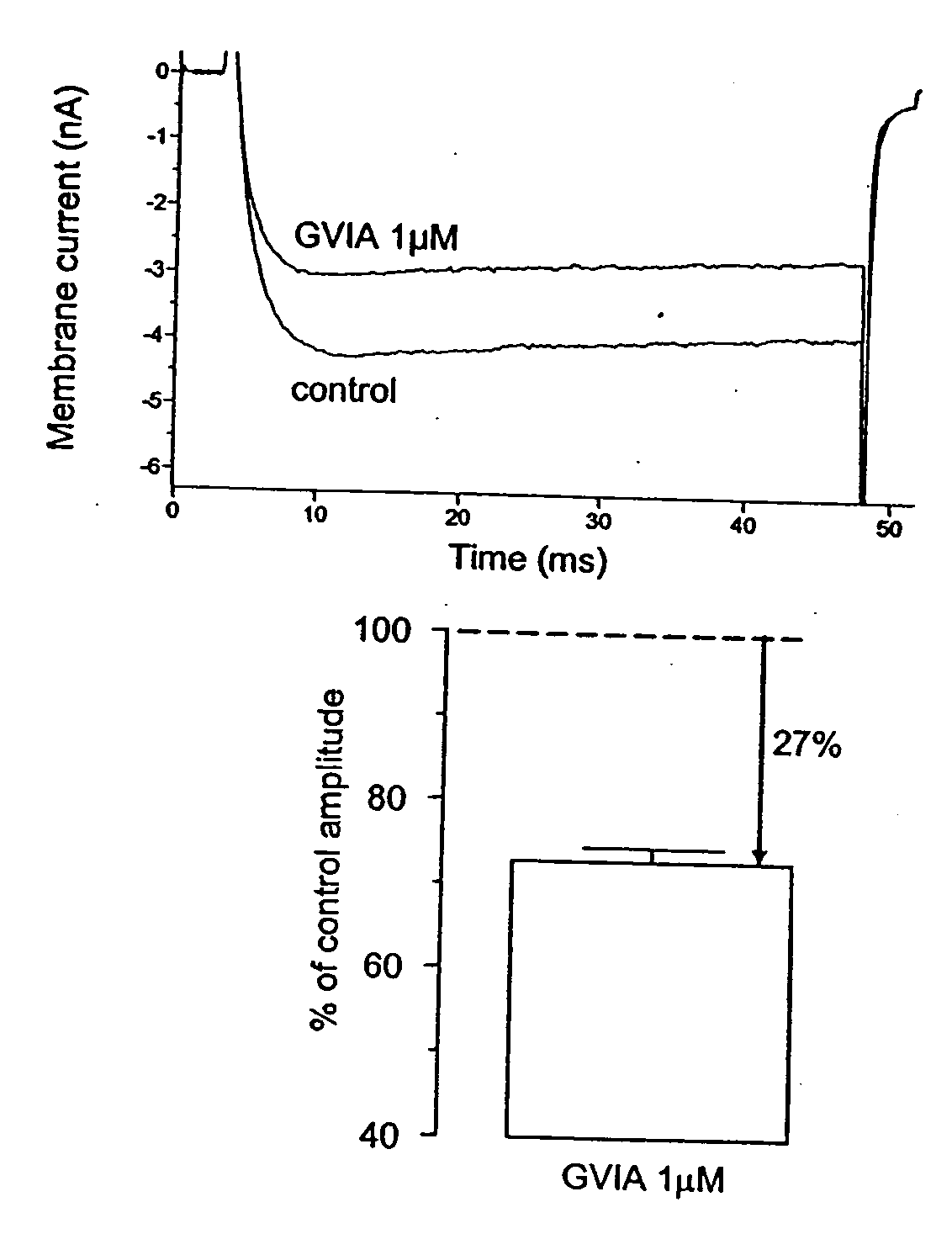
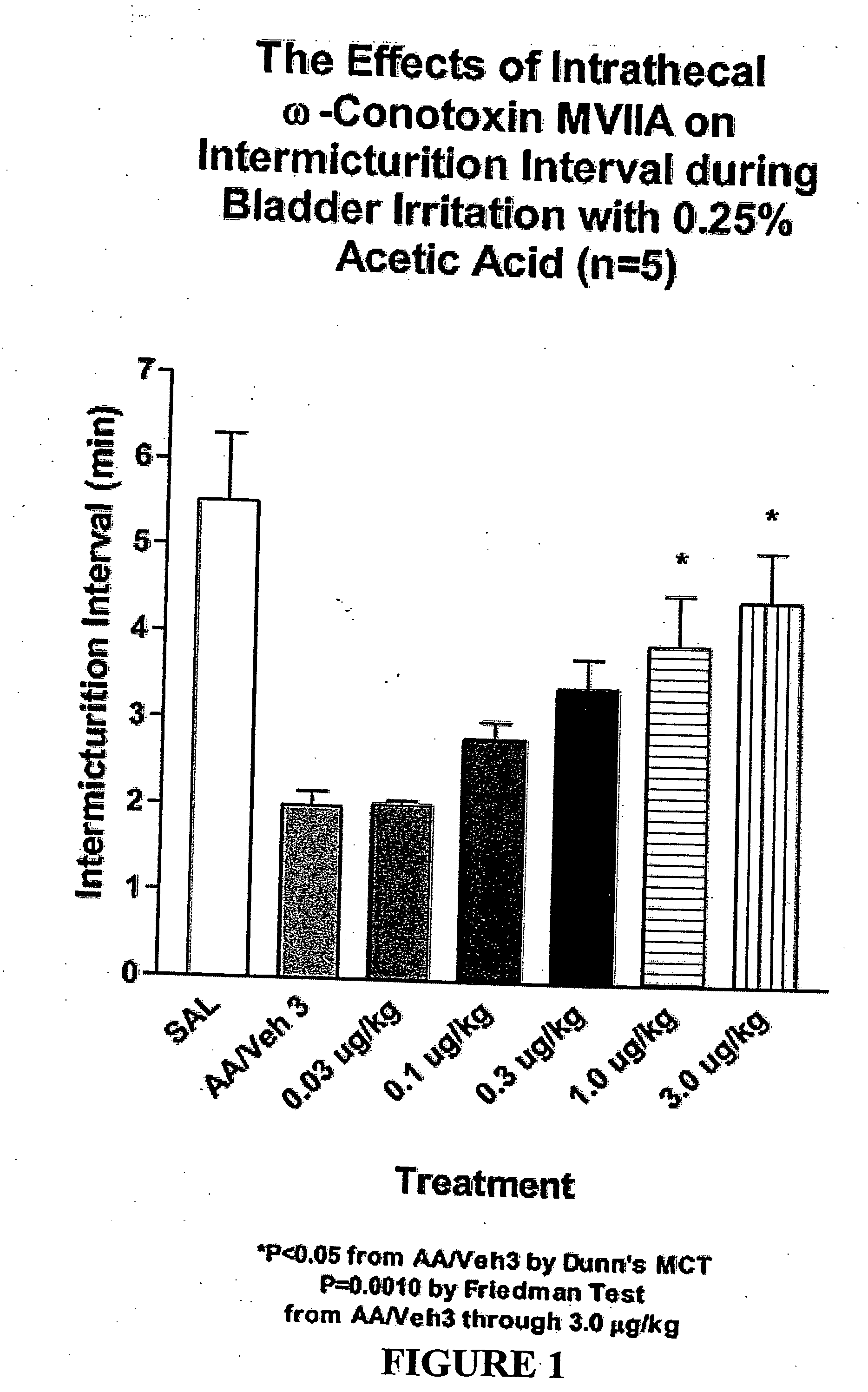
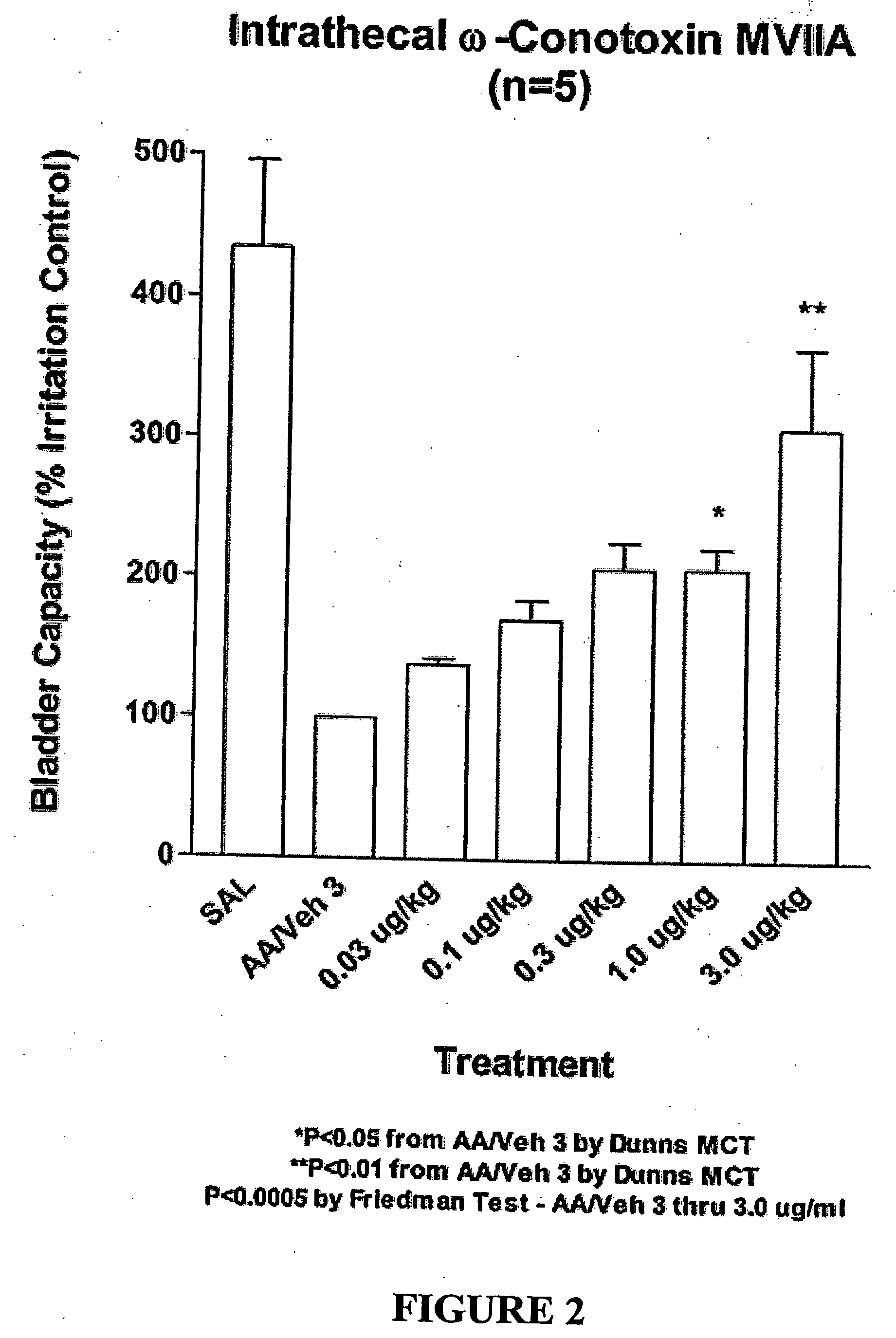
Methods of using ziconotide to treat overactive bladder
The invention relates to methods of using Cav2.2 subunit calcium channel modulators to treat painful and non-painful lower urinary tract disorders and the related genitourinary tract disorders vulvodynia and vulvar vestibulitis in normal and spinal cord injured patients.
Owner:EDUSA PHARMA
Methods of treating lower urinary tract disorders using losigamone
The invention relates to methods of using sodium channel modulators, preferably Losigamone or a pharmaceutically acceptable salt, enantiomer, analog, ester, amide, prodrug, metabolite, or derivative thereof, to treat painful and non-painful lower urinary tract disorders, particularly painful and non-painful overactive bladder with and / or without loss of urine.
Owner:DYNOGEN PHARM INC
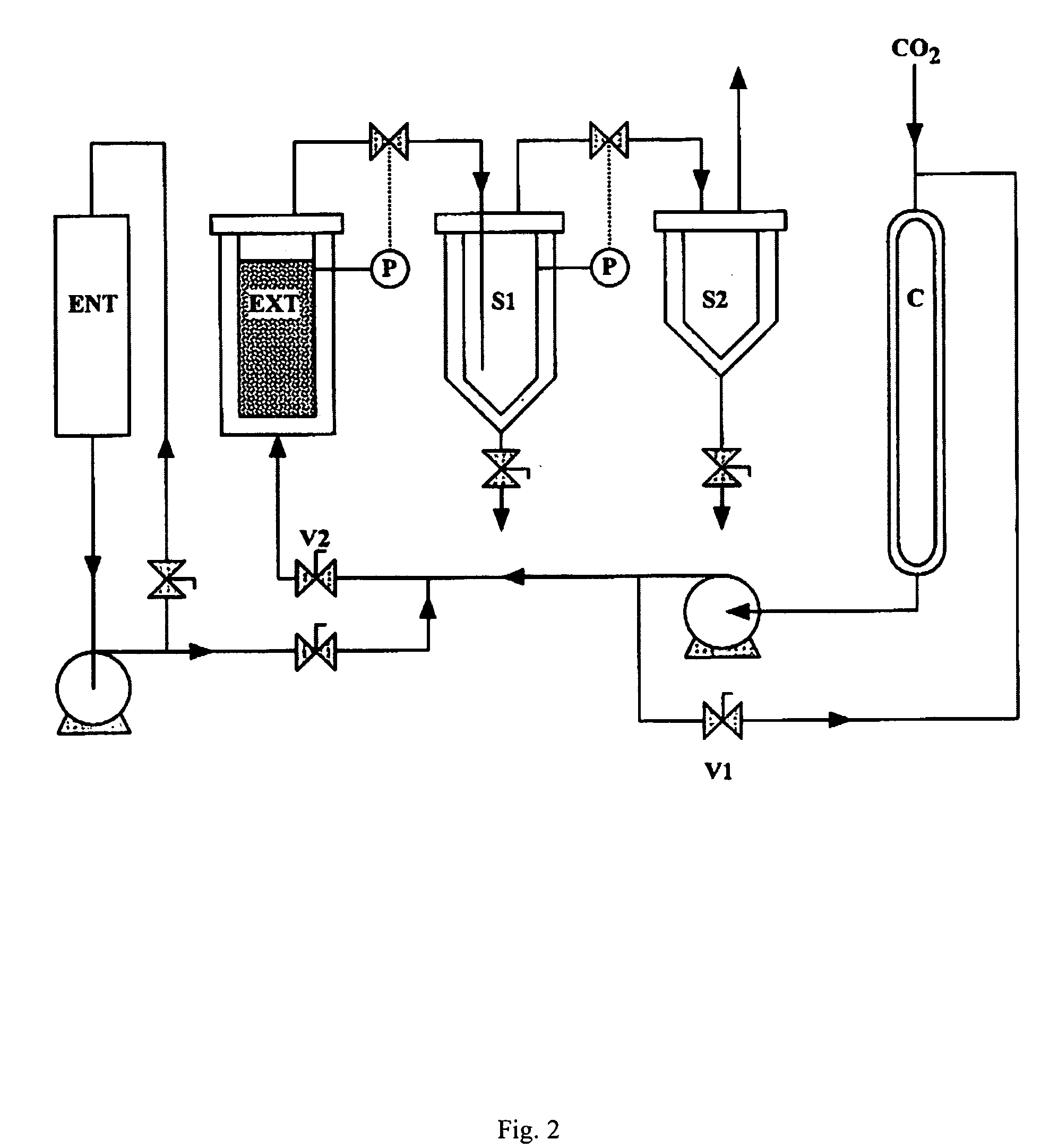
Bacterial extract for digestive or urinary tract disorders and process for its preparation
ActiveUS8236522B2Extend the validity periodEfficient digestionAntibacterial agentsBiocideBacteroidesMedicine
The present invention relates to an extract from bacterial strains useful as a treatment for disorders such as digestive or urinary tract disorders, compositions comprising the extract, and processes of making the extract from media that do not pose a risk of prion diseases.
Owner:OM PHARMA SA
Methods and systems for performing a medical procedure
Method and system for treating a patient using a compressible, pressure-attenuating device. According to one embodiment, the system is used to treat urinary tract disorders and can include one or more of an access device, a delivery device, a pressure-attenuating device, and a removal device. The access device may be used to create a passageway to an anatomical structure, such as the patient's bladder. The delivery device may be inserted through the passageway created by the access device and may be used to deliver the pressure-attenuating device to the anatomical structure. The removal device may be inserted through the passageway created by the access device and may be used to view the bladder and / or to capture, to deflate and to remove the pressure-attenuating device.
Owner:SOLACE THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com