Medicinal composition for treating renal diseases and pharmaceutical use of medicinal composition
A kind of technology of kidney disease and composition, applied in the field of composition for treating kidney disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
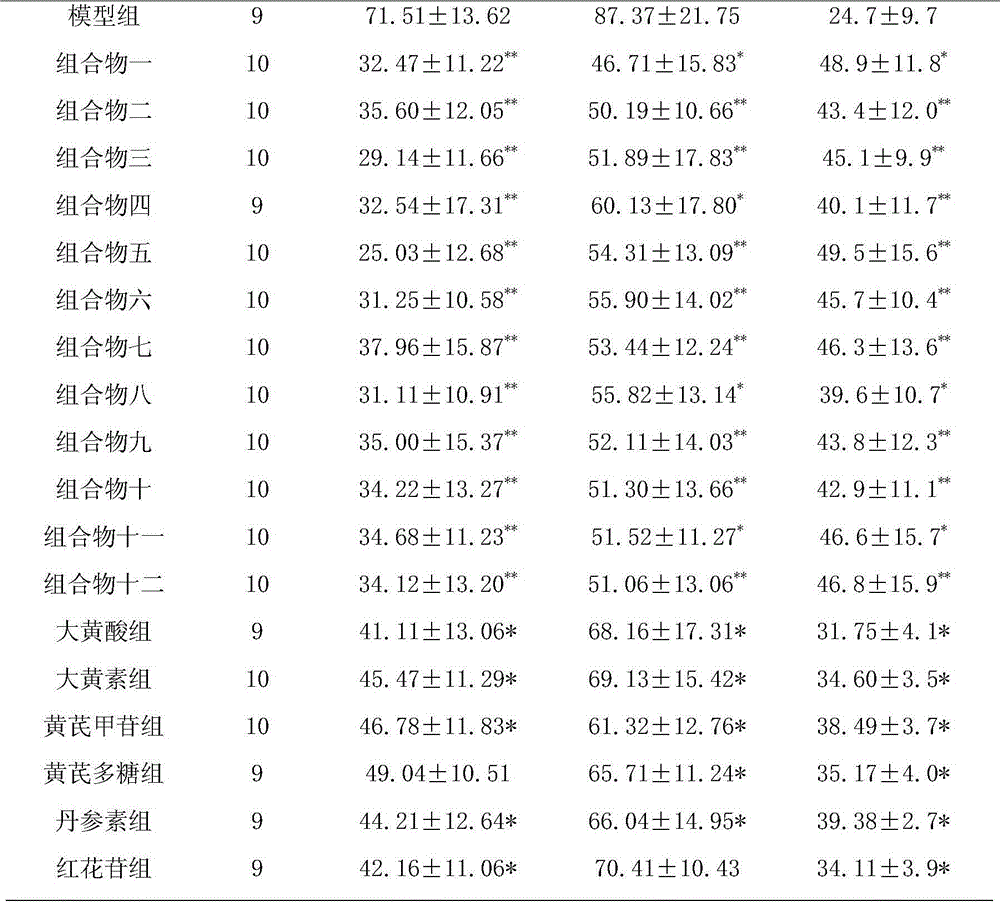
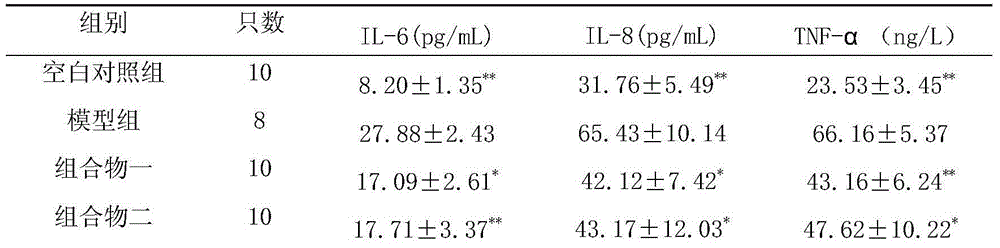
[0138] Take 0.5g of rhein, 0.4g of emodin methyl ether, 0.4g of emodin glucoside, 0.4g of chrysophanol glucoside, 0.5g of danshensu, 0.2g of salvianolic acid B, 0.4g of hydroxyl safflower yellow pigment A, Glycoside 0.2g, astragalus polysaccharide 0.4g, salvianolic acid A0.1g, verbascoside glycosides 0.4g, add PEG-20050ml, add water for injection to dilute to 500ml, filter, add water for injection to 800ml, adjust the pH value to 6.5- 7.0, add water for injection and dilute to 1000ml, filter through a 0.22μm microporous membrane, subpackage, and sterilize to obtain an injection.
Embodiment 2
[0140] Take 50g rhein, 40g emodin methyl ether, 40g chrysophanol glucose sugar, 40g emodin glucoside, 50g salvianol, 20g salvianolic acid B, 40g hydroxyl safflower yellow A, 20g astragaloside IV, 40g astragalus polysaccharide, salvianol Acid A10g, acteocoside 40g, appropriate amount of meglumine and arginine, add water for injection to dilute to 5L, stir until dissolved, filter, adjust the pH value of the filtrate to 5.0-7.0, and use a 0.22μm microporous membrane for fine filtration ; Take an appropriate amount of mannitol and add water for injection to prepare a solution, mix with the above filtrate, filter with a 0.22 μm microporous membrane, add water for injection to dilute to 10L, pack according to the dosage of 10ml of the unit preparation, freeze-dry, and obtain freeze-dried pink.
Embodiment 3
[0142]Take 15.6g of rhein, 12.5g of emodin methyl ether, 12.5g of chrysophanol glucose sugar, 12.5g of emodin glucoside, 15.6g of salvia miltiorrhiza, 6.5g of salvianolic acid B, 12.5g of hydroxyl safflower yellow pigment A, astragalus Glycoside 5.2g, astragalus polysaccharide 10.4g, salvianolic acid A3.5g, verbascoside 12.5g, pre-crossed starch, micropowder silica gel, magnesium stearate, talcum powder, pass through 80 mesh sieve respectively, and mix with the powder evenly , add an appropriate amount of binder to make a soft material, granulate, and dry the granules at 60°C. Add micropowder silica gel, magnesium stearate and talcum powder, mix evenly, and press into 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com