Method for preparing formamide by catalytic oxidation of tertiary amine
A technology of catalytic oxidation and formamide, applied in the field of chemical industry, can solve the problems of difficult product separation and purification, low atom economy, etc., and achieve the effect of high atom economy and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 0.01g MnO 2 Catalyst, 1mmol N,N-dimethylaniline and 2g chlorobenzene were added to a stainless steel autoclave with a polytetrafluoroethylene lining inside. The temperature was raised to a reaction temperature of 100° C. using an automatic temperature controller, and 0.6 MPa oxygen was added to react for 4 hours. During the reaction, the pressure was kept constant. The reaction product was analyzed by GC-MS, the conversion rate of tertiary amine was 92%, and the selectivity of formamide product was 91%.
Embodiment 2-23
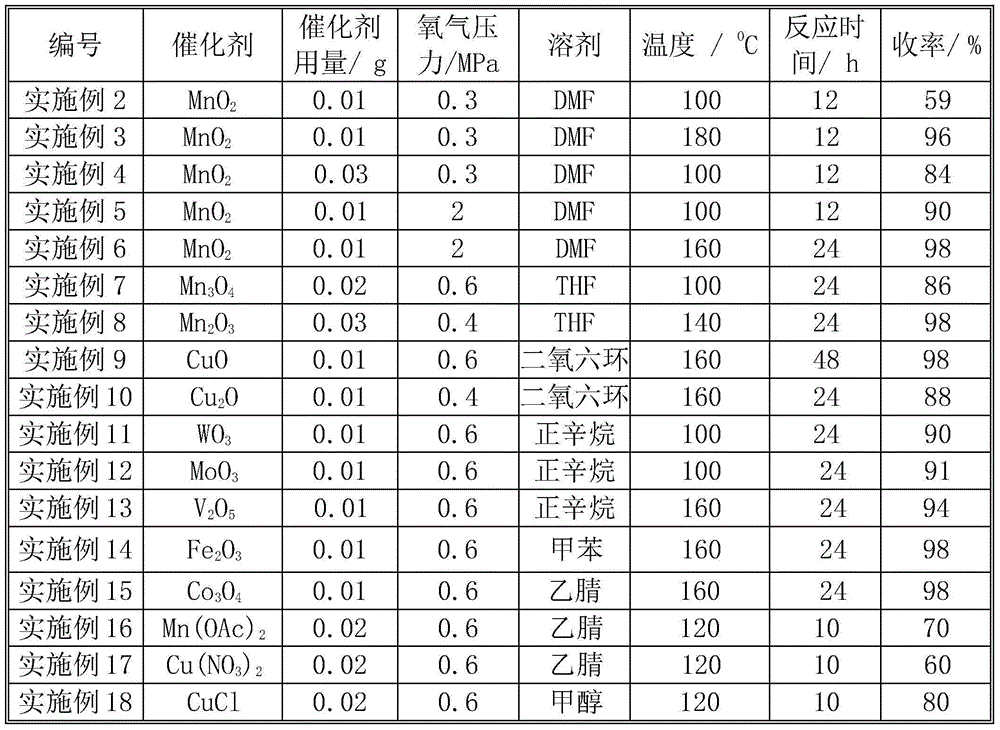
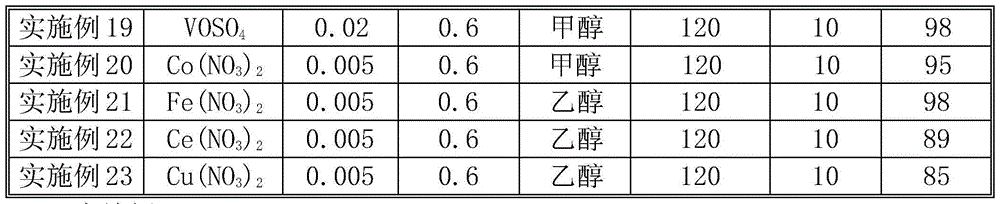
[0016] Except catalyst, consumption, pressure, reaction time are different, catalyst activity evaluation is identical with embodiment 1. The reaction conditions and catalytic reaction results are shown in Table 1. It can be seen from Table 1 that the amount of catalyst, oxygen pressure, reaction temperature and reaction time have an impact on the catalytic effect. With the increase of reaction temperature (Examples 2 and 3), catalyst consumption (Examples 2 and 5), oxygen pressure (Examples 3 and 5) and reaction time (Examples 5 and 6), the yield increased. As the amount of catalyst increases, the oxygen pressure increases, and the yield of azobenzene also increases. Oxides or metal salts of transition metals V, Fe, Co, Mn, Cu, Cr, Mo, Ce, Nb, W, etc., all show good results.
[0017] Table 1 Oxidation of aniline to prepare azobenzene
[0018]
[0019]
Embodiment 24-40
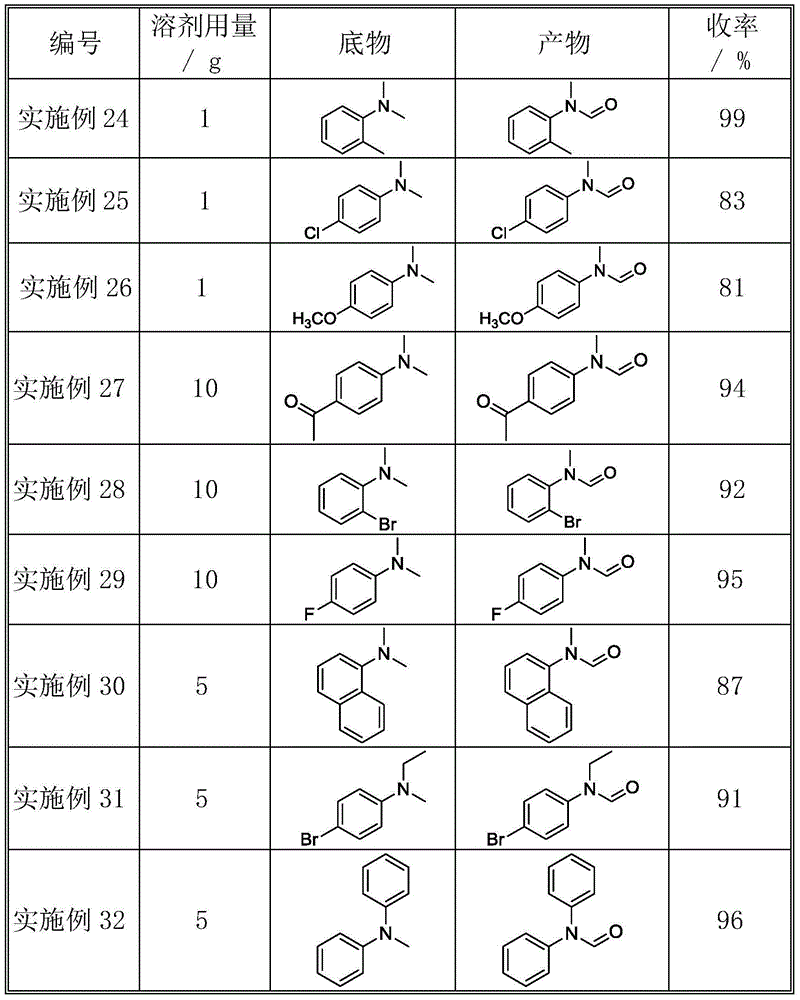
[0021] The catalyst activity evaluation was the same as in Example 1 except that the amount of substrate and solvent was different. The reaction conditions and catalytic reaction results are shown in Table 2. The combination of tertiary amines substituted by different alkyl groups (including aromatic rings, aliphatic chains, and aliphatic ring alkyl groups) can be oxidized into corresponding formamide compounds.
[0022] Table 2 Oxidation of different tertiary amine substrates
[0023]
[0024]
[0025] The invention has high oxidation efficiency and high product yield; uses air or oxygen as the oxygen source, is economical, environmentally friendly and has good application prospects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com