Imidazopyridine compound and preparation method and application thereof
A technology of imidazopyridines and compounds, applied in the field of compounds with skeleton structures, can solve problems such as less NEK2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of embodiment 1 formula (1) imidazopyridine compound
[0025] (1) Synthesis of Fragment I:
[0026] Synthesis of compound B: 5.0 g of A and 4.89 g of NaHCO3 were dissolved in 50 mL of ethanol solution, and 12 mL of chloroacetaldehyde (purity 40%) was added to the mixture. The mixture was heated to reflux for 6 hours. After the reaction, filter the reaction solution with diatomaceous earth, evaporate the organic solvent to dryness, then dilute the residue with 50 mL of ethyl acetate, wash the organic phase twice with 56 mL of water, and extract the obtained aqueous phase twice with 40 mL of ethyl acetate. The obtained organic phase was washed with saturated brine and dried over anhydrous Na2SO4. The dried organic phase was concentrated and then separated by silica gel column chromatography. The eluent was ethyl acetate:petroleum ether=1:1, and 4.44 g of white solid B was finally obtained with a yield of 78%.
[0027] 1 H NMR (400MHz, CDCl 3 )δ7.98(d, J=7.1...
Embodiment 2
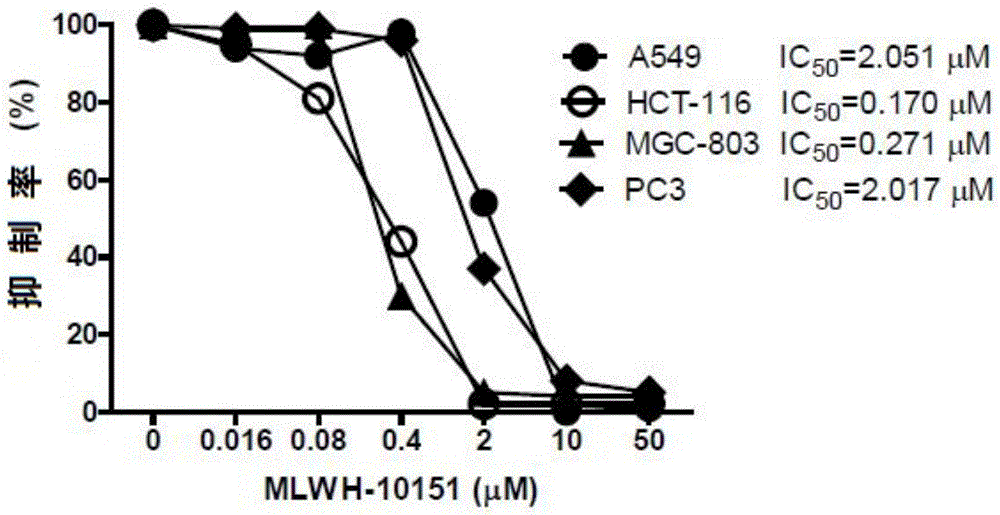
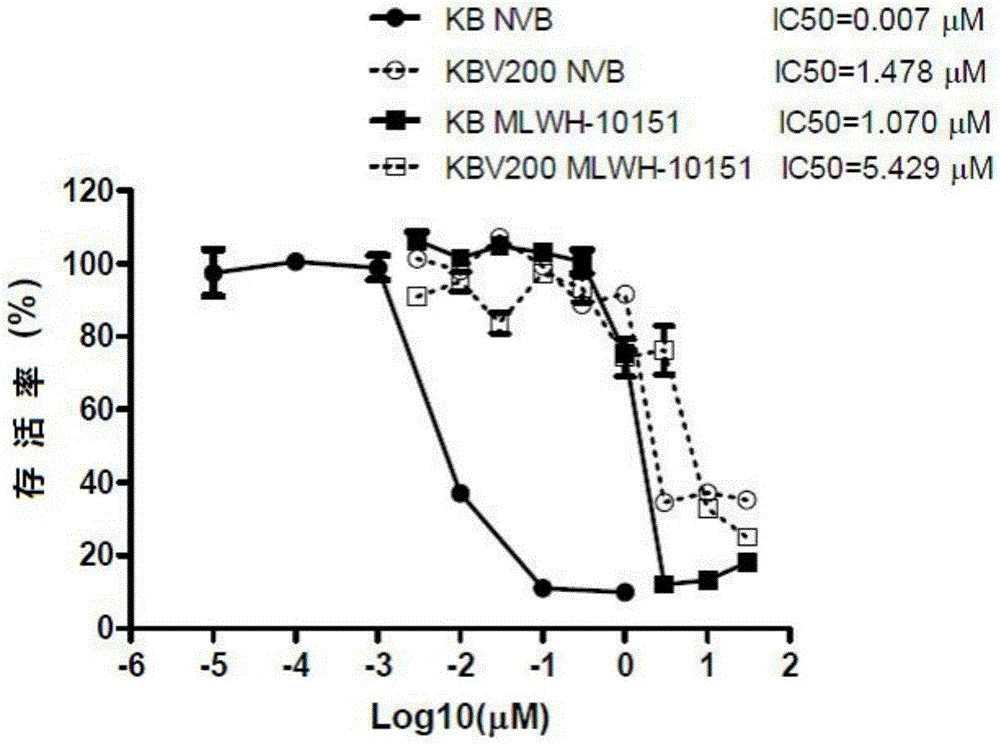
[0045] The in vitro antitumor activity evaluation of embodiment 2 formula (1) imidazopyridine compound
[0046] The research at the cell level of the present invention shows that the imidazopyridine compound (MLWH-10151) of formula (1) can be used as a selective inhibitor of NEK2.
[0047] (1) Inhibitory effect of formula (1) imidazopyridine compound (MLWH-10151) on tumor cell proliferation
[0048] Experimental method: tumor cells in the logarithmic growth phase were inoculated into 96-well culture plates, and different concentrations of MLWH-10151 (0, 0.016, 0.08, 0.4, 2, 10, and 50 μM) were given after the cells adhered to the wall. °C, 5% CO 2 After culturing under the conditions for 72 hours, add 5 mg / mL MTT solution to continue culturing for 4 hours, and add triple solution (10% SDS-5% isobutanol-0.01mol / L HCl) in a CO2 incubator overnight. After the formazan is completely dissolved, measure its absorbance at 570nm with a microplate reader, and calculate the cell viabi...
Embodiment 3
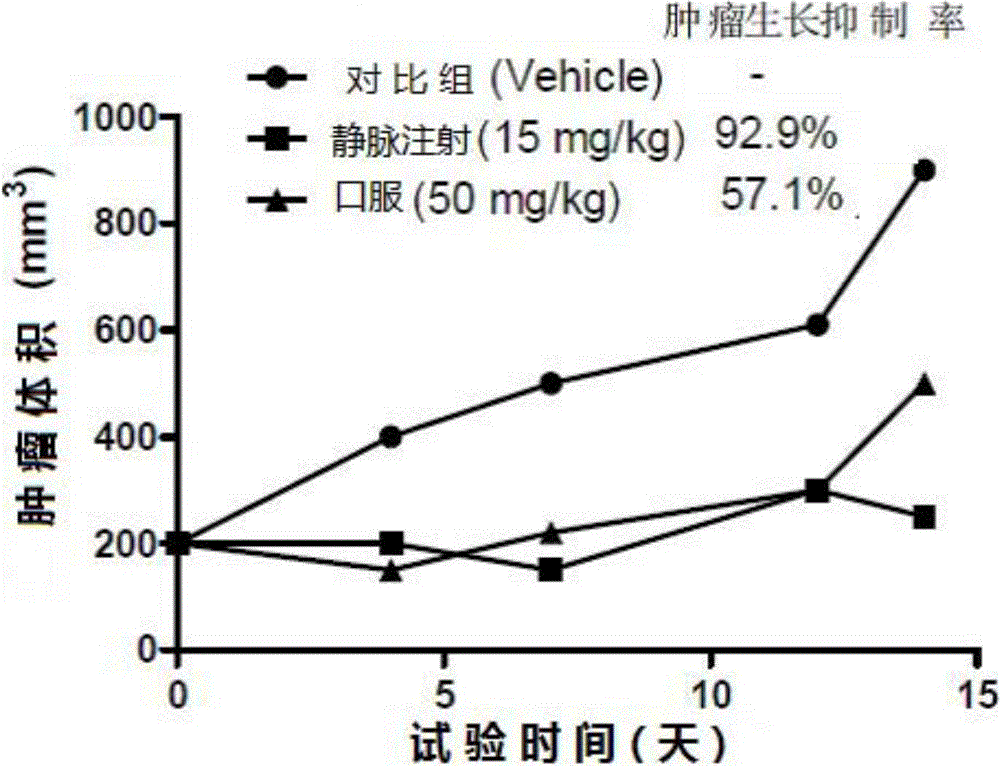
[0051] Embodiment 3 Formula (1) in vivo tumor growth regulation experiment of imidazopyridine compound
[0052] Take PC3 cells in the logarithmic growth phase and inoculate them in the armpit of nude mice until the tumor grows to 100mm 3 At the same time, it was administered orally (50 mg / kg) and intravenously (15 mg / kg), and the body weight and tumor volume of the mice were regularly recorded. like image 3 (Inhibitory effect of MLWH-1015 on xenograft mouse model tumors) shows that the two administration methods can effectively inhibit tumor growth, and the tumor growth inhibition rates (TGI) of oral and intravenous injection groups are 57.1% and 92.9%, respectively. %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com