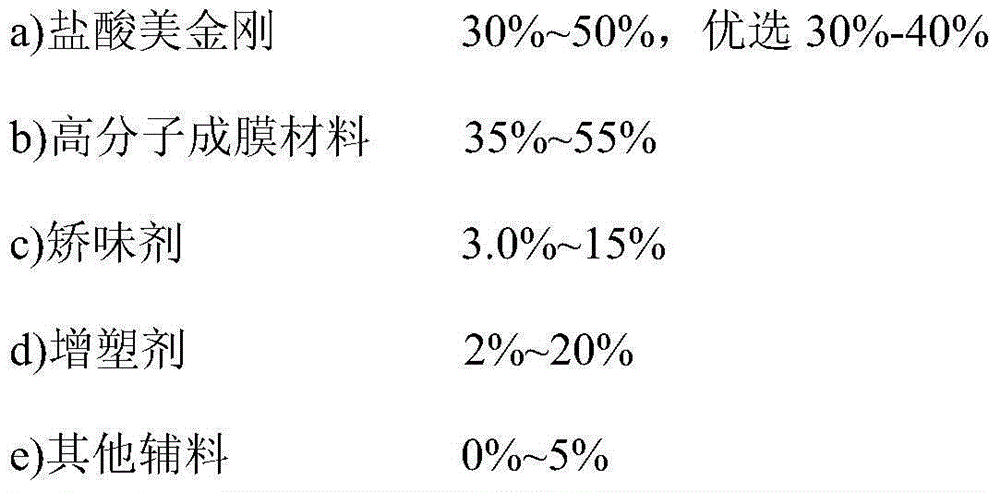
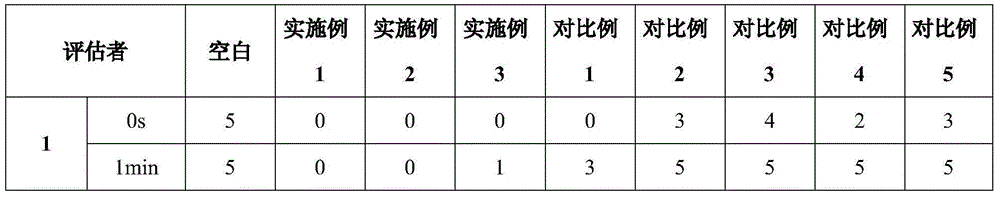
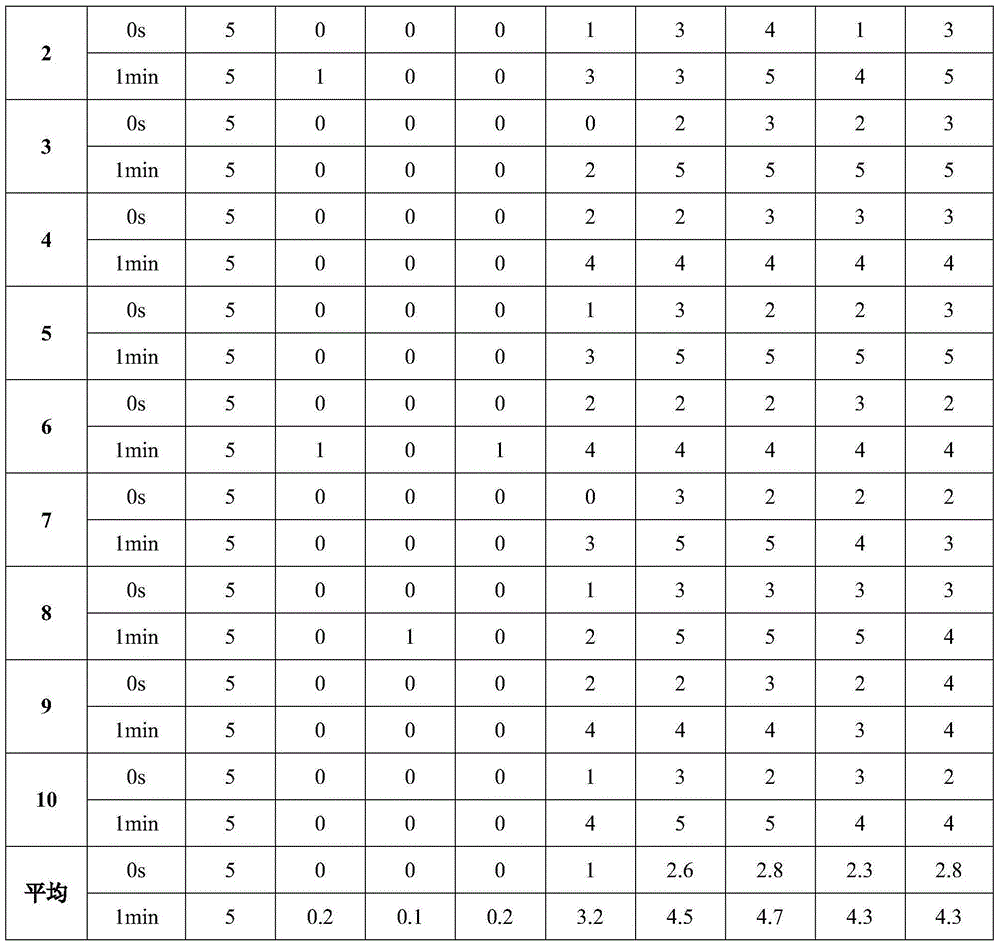
Memantine hydrochloride oral-dissolving film preparation and preparation method and application of preparation
A technology of memantine hydrochloride and oral dissolving film, applied in the field of medicine, can solve the problems of not easy to dissolve quickly, large film thickness, poor patient compliance, etc., and achieve the effects of good taste, smooth surface, and improved patient compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In this example: 1) Anhydrous citric acid and sucralose are used as flavoring agents, which can effectively mask the bitterness and numbness; 2) The content of the main ingredient is 30%, and the prepared film has no ice flower-like dots on the surface; 3 ) The average particle size of the raw material is less than 12 μm, and the prepared film has a smooth surface without roughness; 4) The film-forming material adopts hypromellose, which has good film-forming properties; 5) Bright blue lake is used as a colorant, and the dosage is 0.1% , good appearance.
[0038] The prescription quantity is 600 tablets (specification 5mg) or 300 tablets (specification 10mg)
[0039] prescription:
[0040] Memantine hydrochloride: 3.0g (30%)
[0041] Hypromellose: 5.5g (55%)
[0042] Titanium dioxide: 0.3g (3%)
[0043] Glycerin: 0.7g (7%)
[0044] Anhydrous citric acid: 0.2g (2%)
[0045] Sucralose: 0.3g (3%)
[0046] Bright blue lake: 0.01g (0.1%)
[0047] Purified water: 25.0...
Embodiment 2
[0050] In the present embodiment, compared with embodiment 1: 1) increase strawberry essence as fragrance agent, consumption 2%, taste is good; 2) film-forming material adopts polyvinylpyrrolidone, consumption 52%, film-forming property is good; 3) bright The blue lake is used as a coloring agent, the dosage is 1%, and the appearance is good.
[0051] The prescription quantity is 600 tablets (specification 5mg) or 300 tablets (specification 10mg)
[0052] prescription:
[0053] Memantine hydrochloride: 3.0g (30%)
[0054] Polyvinylpyrrolidone: 5.2g (52%)
[0055] Titanium dioxide: 0.3g (3%)
[0056] Glycerin: 0.7g (7%)
[0057] Anhydrous citric acid: 0.2g (2%)
[0058] Sucralose: 0.3g (3%)
[0059] Bright blue lake: 0.1g (1%)
[0060] Strawberry essence: 0.2g (2%)
[0061] Purified water: 25.0g
[0062] Weigh 3.0g of memantine hydrochloride and add it into 25.0g of purified water, stir and disperse evenly, then weigh 0.2g of anhydrous citric acid, 0.3g of sucralose, 0...
Embodiment 3
[0064] In the present embodiment, compared with embodiment 2: 1) increase strawberry essence as aromatic agent, consumption 4%, taste is good; 2) film-forming material adopts polyoxyethylene, consumption 51%, film-forming property is good; 3) bright Blue starch is not added.
[0065] The prescription quantity is 600 tablets (specification 5mg) or 300 tablets (specification 10mg)
[0066] prescription:
[0067] Memantine hydrochloride: 3..0g (30%)
[0068] Polyoxyethylene: 5.1g (51%)
[0069] Titanium dioxide: 0.3g (3%)
[0070] Glycerin: 0.7g (7%)
[0071] Anhydrous citric acid: 0.2g (2%)
[0072] Sucralose: 0.3g (3%)
[0073] Strawberry essence: 0.4g (4%)
[0074] Purified water: 25.0g
[0075] Weigh 3.0g memantine hydrochloride and add it into 25.0g purified water, stir and disperse evenly, then weigh 0.2g anhydrous citric acid, 0.3g sucralose, 0.3g titanium dioxide, 0.7g glycerin, 0.4g strawberry essence, add purified In water, stir and disperse evenly, weigh 5.1g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Breaking force | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com