Preparation method and application of HLA-A0201-restrictive anti-Sox2 specific CTL
A specific and restrictive technology, applied in the field of genetic engineering, can solve the problems of low antigen uptake, poor anti-tumor treatment effect, insufficient killing activity of CTL cells, etc., and achieves simple and easy preparation method, good stability and convenience The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 This example discloses the preparation of a C-type lectin-type sugar-modified cationic liposome antigen carrier. The specific operations are as follows:
[0049] (1) First prepare mannose or mannoside modified polyethylene glycol derivatized phosphatidylethanolamine phospholipids. Mannose or mannoside is connected to the amino group of polyethylene glycol derivatized phosphatidylethanolamine phospholipid through aldehyde-amino or hydroxyl-amino condensation, so as to obtain the polyethylene glycol derivatized phosphatidyl alcohol of mannose or mannoside ethanolamine phospholipids. Then the cationic lipid DOTAP and the polyethylene glycol derivatized phosphatidylethanolamine phospholipid of mannose or mannoside are respectively dissolved in chloroform-methanol (2:1), and mixed according to a certain ratio, and the mixed solution is obtained after the two are mixed. Place in a round bottom flask. Rotate the mixed solution to dryness with a stable rotary evapor...
Embodiment 2
[0050] Example 2 This example discloses the production process of 72-hour rapid maturation of dendritic cells loaded with tumor stem cell antigens, the specific operations are as follows:
[0051] (1) Collect and isolate mononuclear cells from peripheral blood, and mononuclear cells are separated at 3.0-5.0*10 6 Cells / ml suspended in AIM-V serum-free culture medium, then add cytokines IL-4 and GM-CSF DC cell induction medium to the culture medium to induce DC formation, and then culture at 37°C, 5% CO2 Cultured in a box, cultured in saturated humidity for DC cell induction.
[0052] (2) The next day, add cationic liposomes wrapped with tumor stem cell Sox2 antigen (as shown in the sequence listing) to the DC cell culture medium at a concentration of 1-2.5ug / ml and incubate for 6 hours Afterwards, DC maturation-promoting medium was added to the DC cells, and the culture was continued for 24-48 hours to obtain DC cells loaded with the tumor stem cell antigen Sox2.
[0053] Ide...
Embodiment 3
[0054] Example 3 This example discloses the process of 72-hour rapidly maturing DCs inducing the secretion of Sox2CTL cytotoxic cytokines, and the specific operations are as follows:
[0055] (1) Add CTL-inducing factors to DC cells loaded with tumor stem cell antigens, induce for 4-7 days, and add medium containing CTL-inducing factors every two and a half days in the middle.
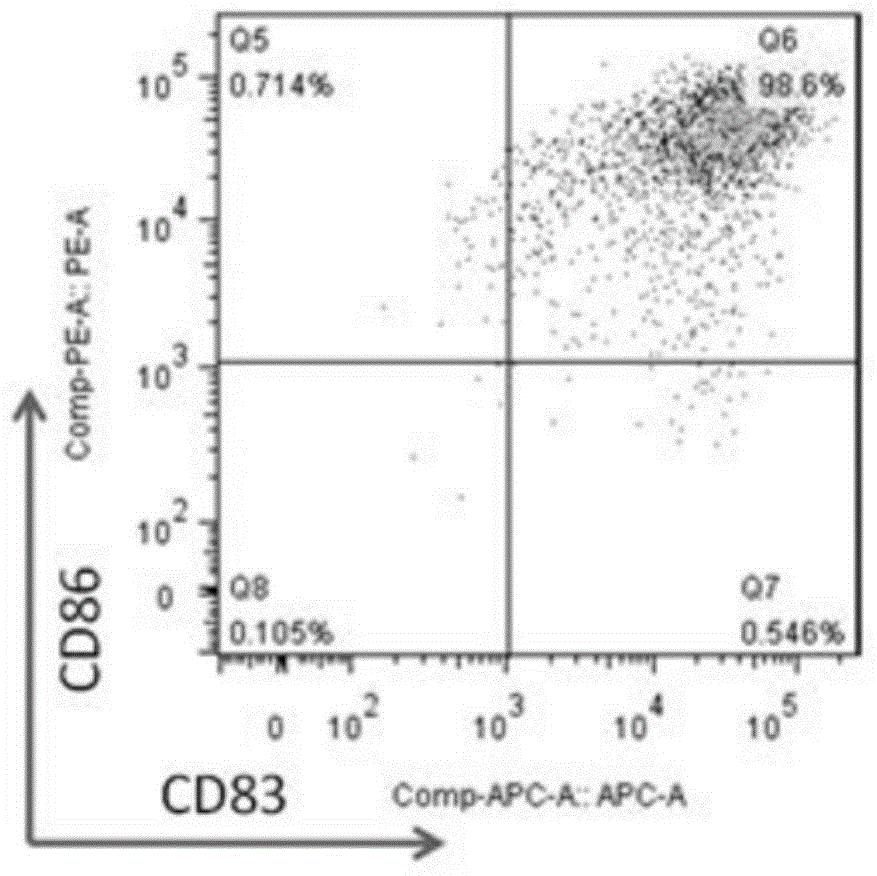
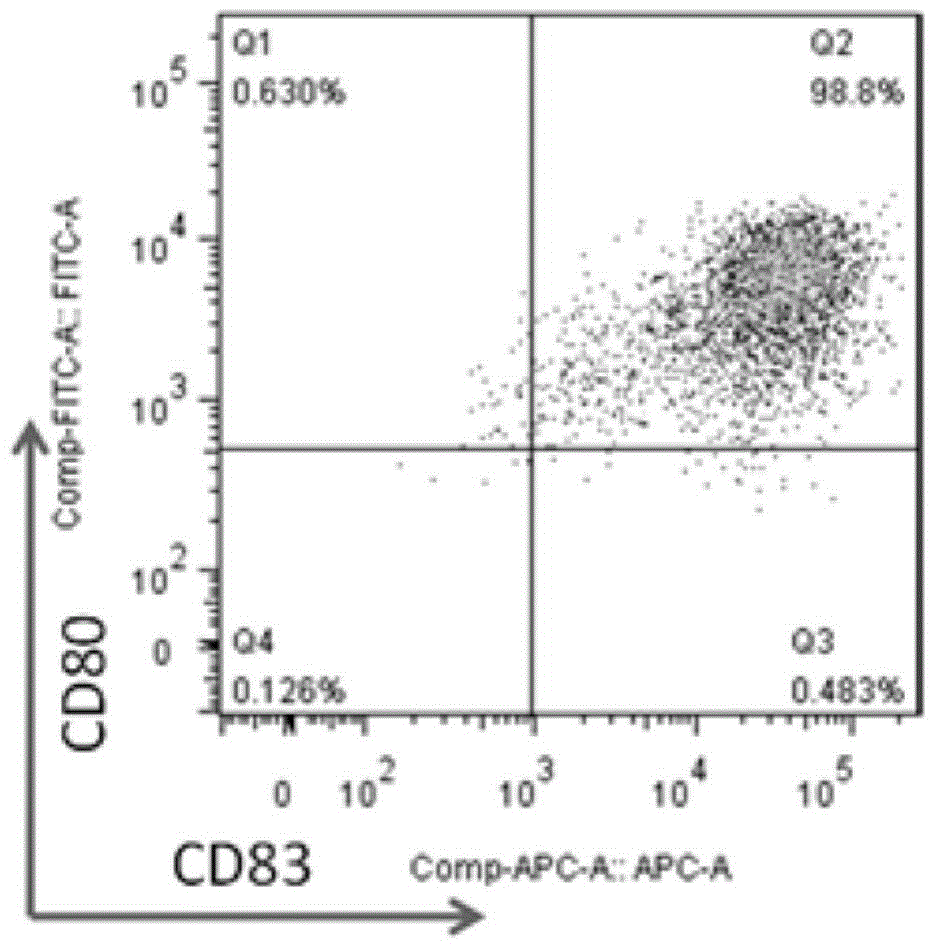
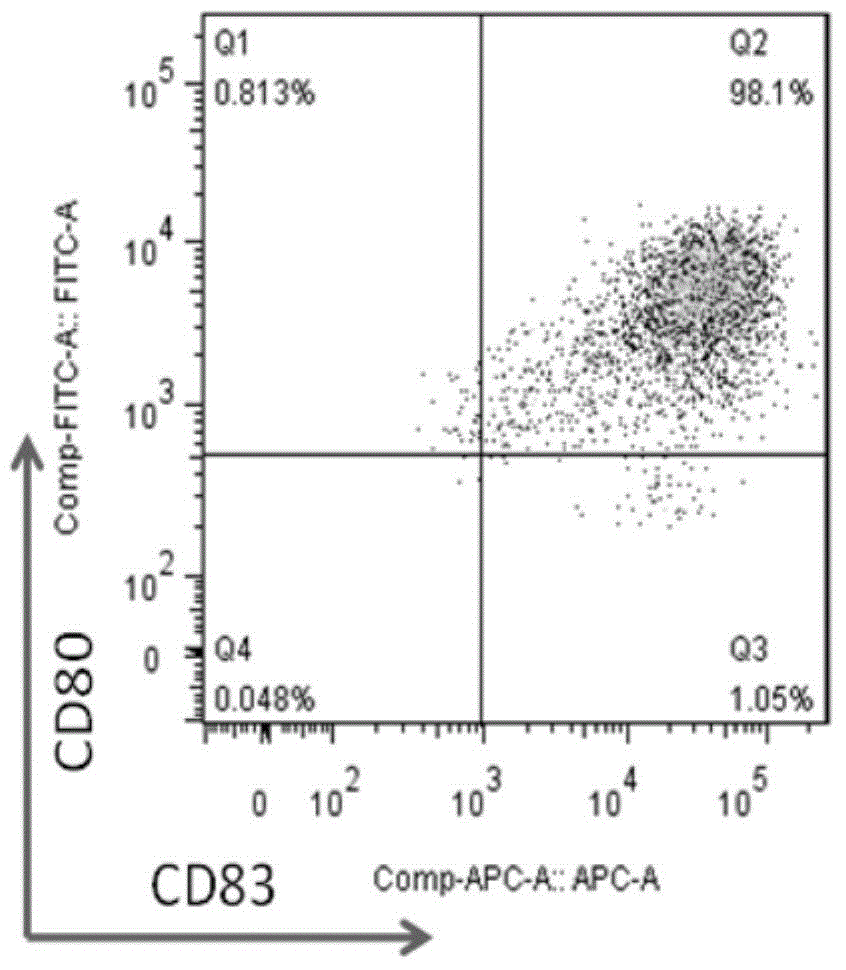
[0056] (2) After CTL induction is completed, add BFA at a concentration of 10 ng / ml. After 4 hours, collect CTL by centrifugation, and use flow cytometry antibody IFN-r and CD8 staining to detect the secretion and expression of cytotoxic factor IFN-r, tumor stem cell antigen Sox2CTL cells Toxicity factor IFN-r test results are as follows: Figure 2A / B / C / D / E / F and Figure 3A / B / C / D / E / F shows:
[0057] Figure 2A / B / C / D / E / F and Figure 3A / B / C / D / E / F, these two sets of figures are a series of pictures of the detection data of the cytotoxic factor IFN-r of the tumor stem cell antigen SOX-2CTL induced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com