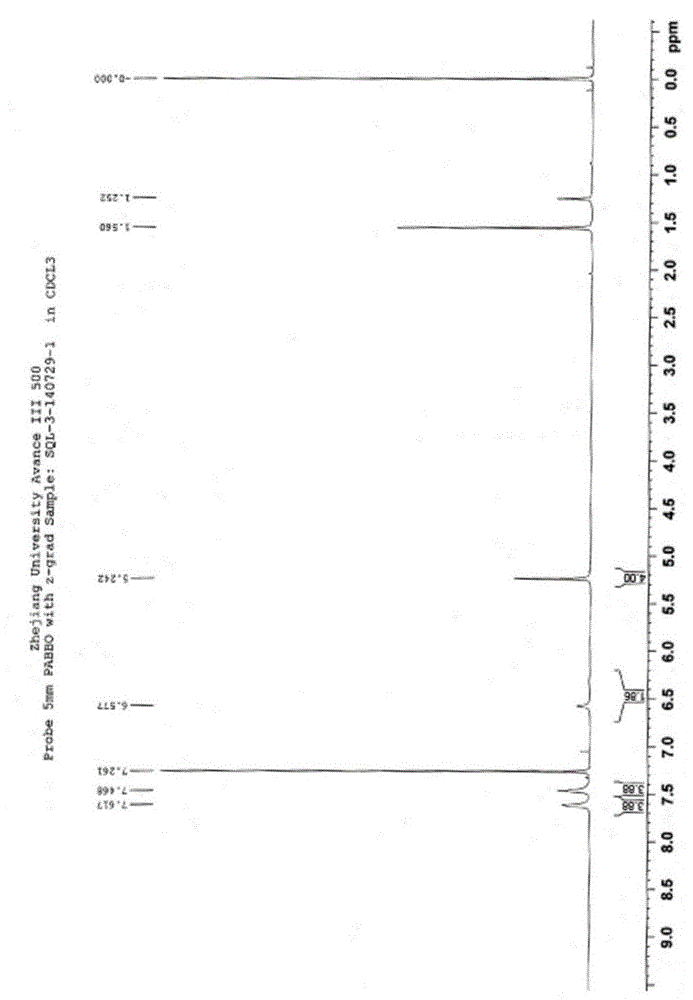
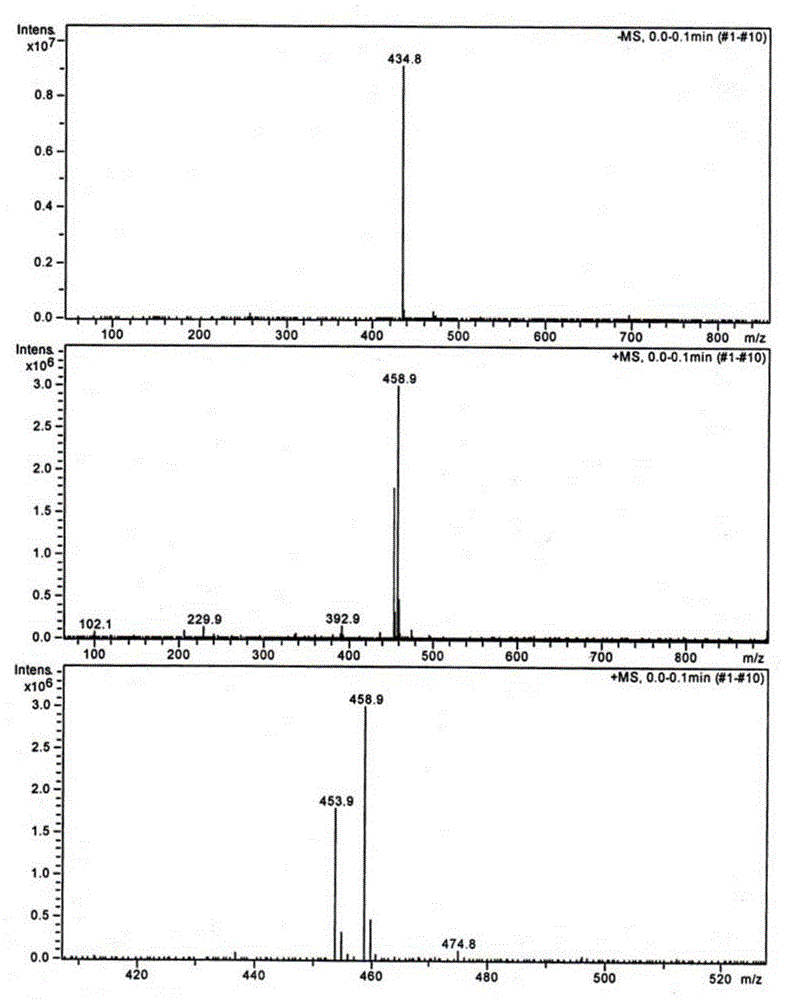
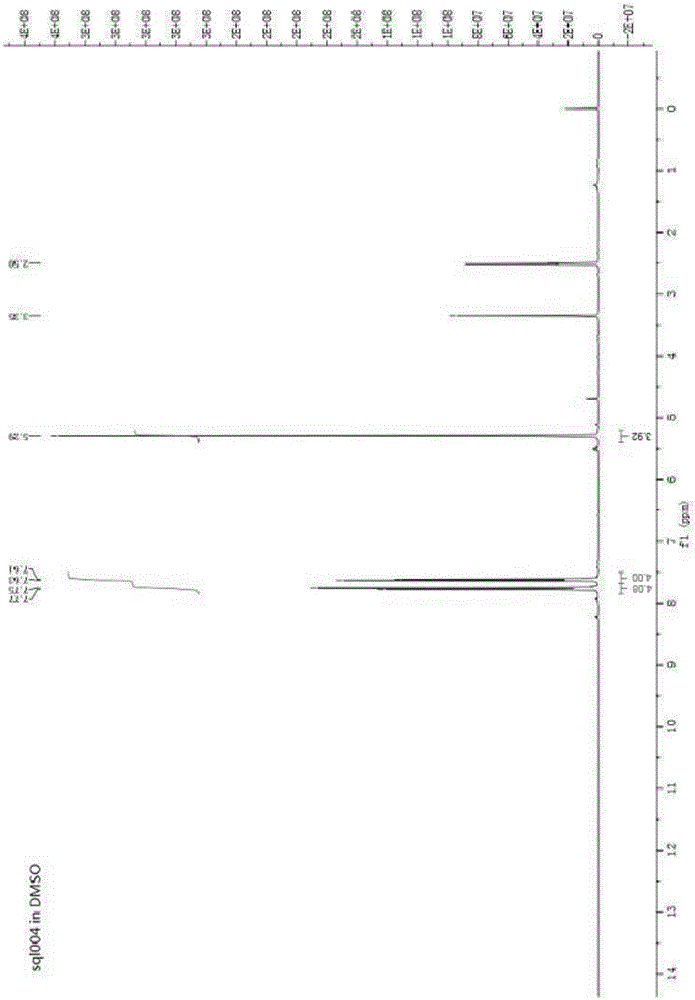
Di(4-trifluoromethylbenzyl) azodiformate, intermediate and preparation method of di(4-trifluoromethylbenzyl) azodiformate
A technology of trifluoromethyl benzyl ester and azodicarboxylic acid, applied in the field of organic compound synthesis, can solve the problems of difficult recovery, difficult separation, limited application research and the like, and achieves the effects of strong practicability, environmental friendliness and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 4-trifluoromethylbenzyl chloroformate
[0042]
[0043] In a 250mL four-necked reaction flask, dissolve triphosgene (14.8g, 50mmol) in 100mL of dichloromethane, and control the temperature below 5°C in an ice bath. 4-Trifluoromethylbenzyl alcohol (8.8 g, 50 mmol) was slowly added dropwise. After the dropwise addition was completed, stirring was continued for 2 h, and then the reaction was continued for 42 h at room temperature. The reaction progress was monitored by TLC. After the reaction is complete, wash with water (100mL×3), the mass fraction is 8% NaHCO 3 Wash with aqueous solution (100mL×2), and finally wash with saturated brine (100mL×1) to obtain a dichloromethane solution of 4-trifluoromethylbenzyl chloroformate, which is directly used in the next reaction.
[0044] Synthesis of Di-4-trifluoromethylbenzyl Hydrazinedicarboxylate
[0045]
[0046] In a 500mL four-necked flask, add the dichloromethane solution of chloroformic acid-4-trifluorom...
Embodiment 2
[0054] Synthesis of 4-trifluoromethylbenzyl chloroformate
[0055] In a 250 mL four-neck reaction flask, dissolve triphosgene (10.4 g, 35 mmol) in 120 mL of chloroform, and control the temperature below 5° C. in an ice bath. 4-Trifluoromethylbenzyl alcohol (8.8 g, 50 mmol) was slowly added dropwise. After the dropwise addition was completed, stirring was continued for 2 h, and then the reaction was continued for 42 h at room temperature. The reaction progress was monitored by TLC. After the reaction is complete, wash with water (100mL×3), the mass fraction is 5% KHCO 3 Wash with aqueous solution (100mL×2), and finally wash with saturated brine (100mL×1) to obtain a chloroform solution of 4-trifluoromethylbenzyl chloroformate, which is directly used in the next reaction.
[0056] Synthesis of Di-4-trifluoromethylbenzyl Hydrazinedicarboxylate
[0057] In the 500mL four-necked flask, add the chloroform solution of 4-trifluoromethylbenzyl chloroformate obtained in the previous s...
Embodiment 3
[0061] Synthesis of 4-trifluoromethylbenzyl chloroformate
[0062] In a 250mL four-necked reaction flask, dissolve triphosgene (13.3g, 45mmol) in 70mL of 1,2-dichloroethane, and control the temperature below 5°C in an ice bath. 4-Trifluoromethylbenzyl alcohol (8.8 g, 50 mmol) was slowly added dropwise. After the dropwise addition was completed, stirring was continued for 2 h, and then the reaction was continued for 42 h at room temperature. The reaction progress was monitored by TLC. After the reaction is complete, wash with water (100mL×3), the mass fraction is 3% KHCO 3 Wash with aqueous solution (100mL×2), and finally wash with saturated brine (100mL×1) to obtain a solution of 4-trifluoromethylbenzyl chloroformate in 1,2-dichloroethane, which is directly used in the next reaction.
[0063] Synthesis of Di-4-trifluoromethylbenzyl Hydrazinedicarboxylate
[0064] In a 500mL four-neck flask, add the 1,2-dichloroethane solution of 4-trifluoromethylbenzyl chloroformate obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com