Process for preparation of methacrylic acid and methacrylic acid esters
A technology of methacrylic acid ester and methacrylic acid, applied in the field of preparation of methacrylic acid, can solve the problems of not teaching the recovery of methacrylic acid, etc., and achieve the effects of good color value and high space-time yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
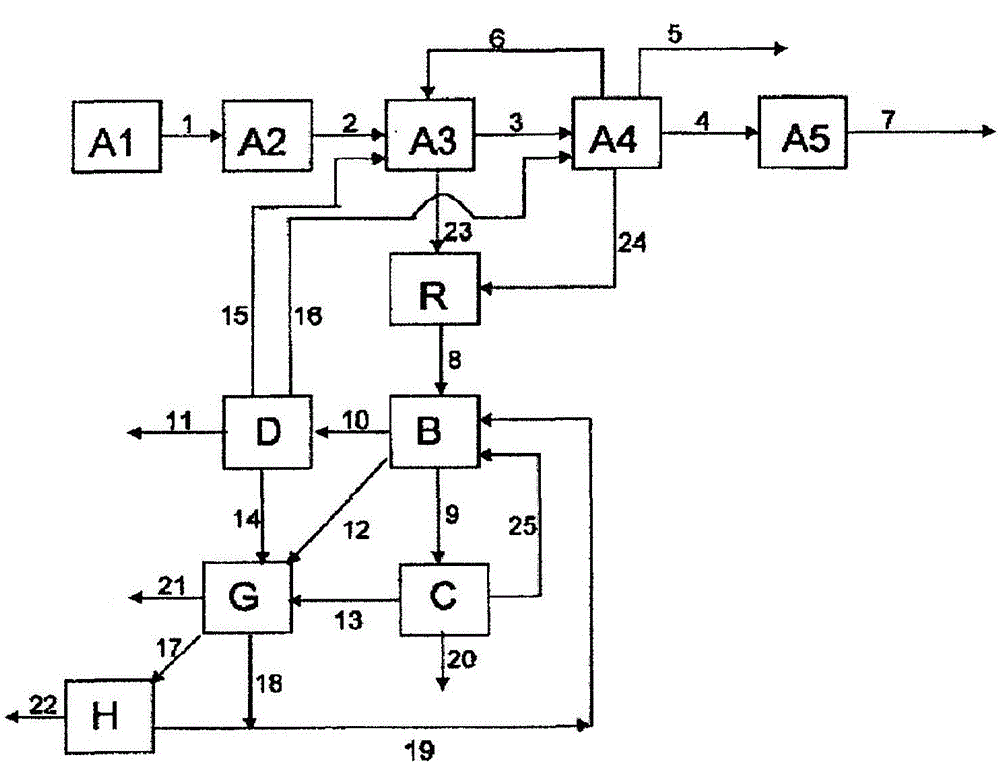
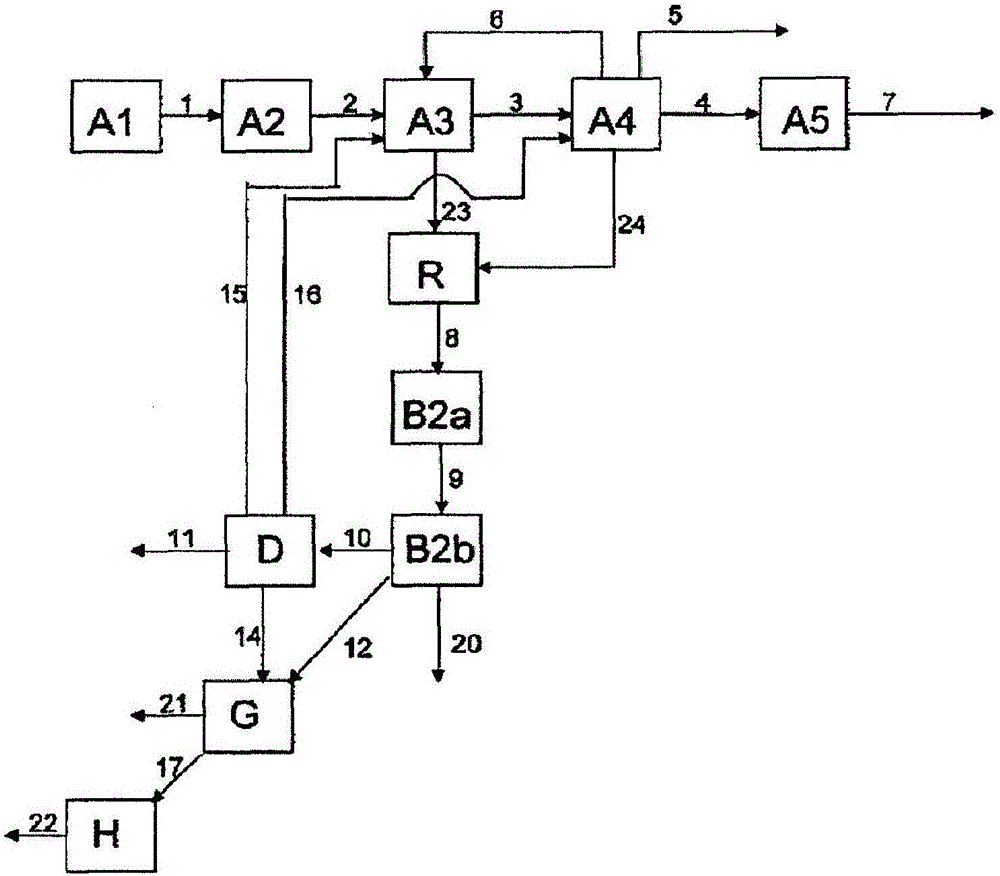
[0238] Example 1 describes how Figure 4 part of the method shown. 82.1% by weight of MAA, 14.3% by weight of various and partially unknown high boilers (dimeric and oligomeric MAA, maleic acid, terephthalic acid, citraconic acid, polymer etc.) and 3.6% by weight of a polymerization inhibitor (mainly hydroquinone) and the first aqueous phase containing 0.6% by weight of MAA and 5.0% by weight of high boilers isolated from the first extraction unit A3 at a ratio of 1:80 The ratio of the high boiler phase to the first aqueous phase is combined in the merging unit R. The resulting concentrations in the combined phase were measured to be 1.6% by weight of MAA and 5.1% by weight of high boilers. The combined phases are extracted in a second separation unit B with n-hexane. 4.9% by weight of MAA was produced in the organic phase, compared to 3.9% by weight of MAA in the comparative case when the first aqueous phase and the high boiler phase were not combined.
Embodiment 2
[0240] In a heatable two-stage reactor for oxidation (diameter: 16 mm) comprising evaporator, salt bath and quench column, the following streams have been fed. Polyphosphoricmolybdenumic acid (composition: Mo(10)V(1)P(1)Cu(0.2)As(0.2)Ce(0.2)) was used as a catalyst. Catalyst loading in the second stage (oxidation to methacrylic acid) was 1580h -1 .
[0241] Stream 1: Methacrolein (MAL) synthesized by aldol condensation reaction with propionaldehyde and formaldehyde as reactant, which contains 0.7% by weight of DIMAL (dipolymethacrolein), 1.5% by weight of water and 0.1 % Propionaldehyde. The stream was evaporated and oxygen, nitrogen and water in a ratio of 2.6:14:7 (reference 1 part MAL) were added to this stream in successive steps.
[0242] Stream 2: MAL synthesized via gas-phase oxidation of tert-butanol has been fed as a gas to the reactor together with oxygen, nitrogen and water. The ratio of MAL to air to nitrogen to water is 1:2.6:14:7.
[0243] For the examples c...
Embodiment 2c
[0256] Example 2c) Mixture of streams 1 and 2 in a ratio (reference MAL) of 1:1:
[0257] DIMAL content: 3300ppm,
[0258] Temperature of the salt bath (for 75% conversion): 311.5°C
[0259] Selectivity to methacrylic acid: 83.8%
[0260] TPA content in quenched liquid: about 400ppm
[0261] Minor clogging in the column; downtime for cleaning required after 25 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com