Synthesis method of dimethyl 2,6-naphthalenedicarboxylate
A technology of dimethyl naphthalate and a synthetic method, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of severe equipment corrosion, long reaction time, and high alkyd ratio, and achieve Reduced reaction time, good selectivity, and the effect of inhibiting etherification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
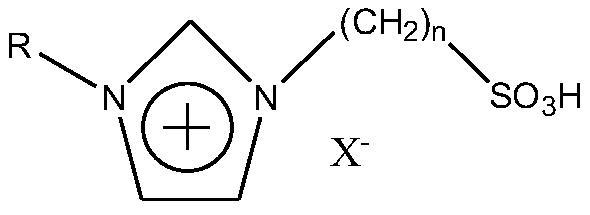
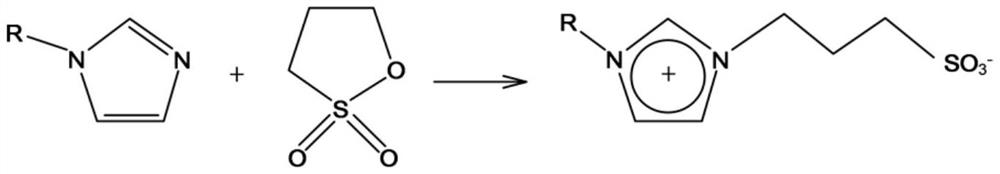
[0024] The ionic liquid synthesis of the present invention can be carried out as follows. The first step is to synthesize the ylide. First, melt 1,3-propane sultone into a liquid state in a water bath at 40°C, then weigh 0.1mol of 1,3-propane sultone into a 250ml three-necked flask, and add about 150ml of ethyl acetate to make it It dissolves. Place the three-necked flask in an ice-water bath. The three-necked flask is equipped with agitator. Turn on the agitator, and then add 0.1 mol of alkyl substituent imidazole dropwise. After the dropwise addition, move the three-necked flask into a water bath and heat to 70°C. The reaction was stirred for 6h. After the reaction was complete, it was filtered with suction, and the obtained solid was washed three times with ethyl acetate, and finally vacuum-dried or rotary evaporated to remove residual ethyl acetate. During the first step of the reaction, the main reactions are as follows:
[0025]
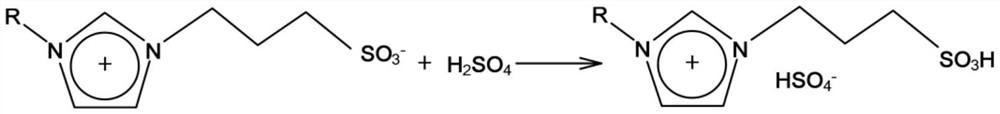
[0026] The second reaction is the...
Embodiment 2
[0034] Adopt embodiment 1 synthetic route to synthesize 120 g of methanol, 12 g of 2,6-NDA, 3.4 g of 2,6-NDC and 1.2 g of the above-mentioned ionic liquid catalyst were added into a 500 ml stainless steel reactor. A packing tower is installed on the top of the stainless steel reaction kettle, and the packing tower is filled with molecular sieves to remove the water generated in the reaction system and make the reaction move in the positive direction. At the beginning of the reaction, the whole system was replaced with nitrogen three times to remove the air in the system, and then pressurized to 1Mpa. Turn on the heating so that the temperature in the kettle rises to 130° C., and at the same time turn on the stirring. When the temperature in the kettle rose to 130°C, the timing was started, and experiments with reaction times of 2h, 3h, and 4h were carried out respectively. The reaction product is pressed into the collection tank, and after the temperature is cooled to 40°C,...
Embodiment 3
[0039] Adopt embodiment 1 synthetic route to synthesize 120 g of methanol, 12 g of 2,6-NDA and 1.2 g of the above-mentioned ionic liquid catalyst were added into a 500 ml stainless steel reactor. The system was replaced with nitrogen three times to remove the air in the system, and then pressurized to 1Mpa. Turn on the heating so that the temperature in the kettle rises to 120° C., and at the same time turn on the stirring. When the temperature in the kettle rose to 120°C, the timer was started, and the reaction was terminated after 5 hours of reaction. The reaction product is pressed into the collection tank, and after the temperature is cooled to 40°C, the pressure is released. The resulting product was filtered and then dried in a vacuum oven.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com