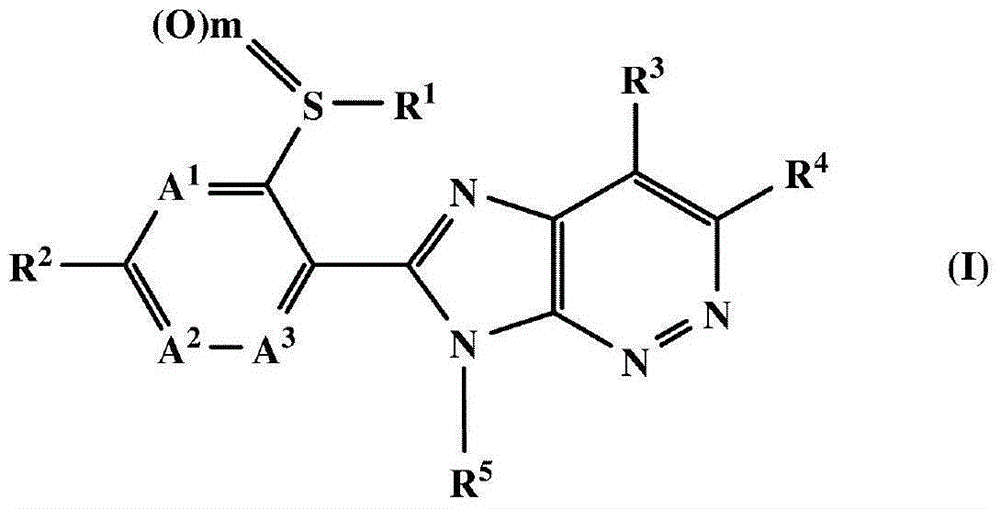
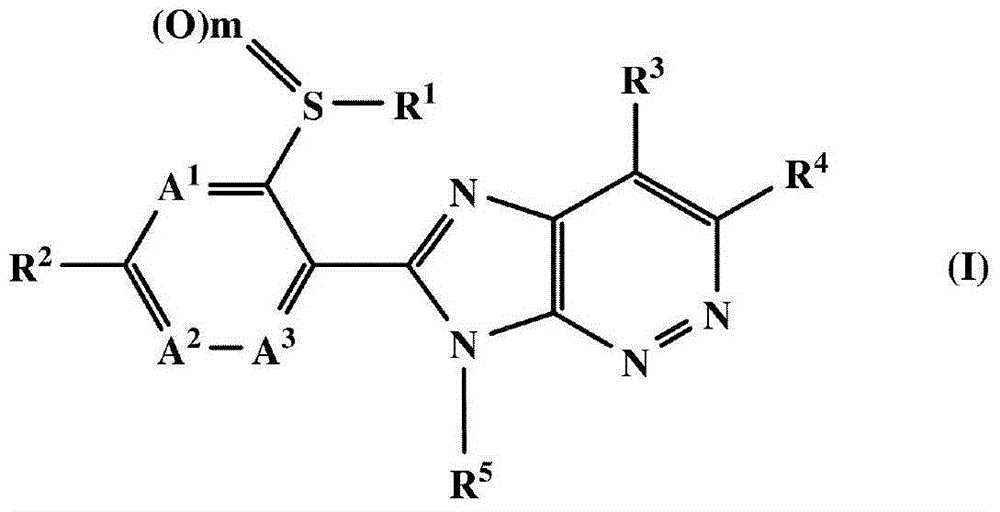
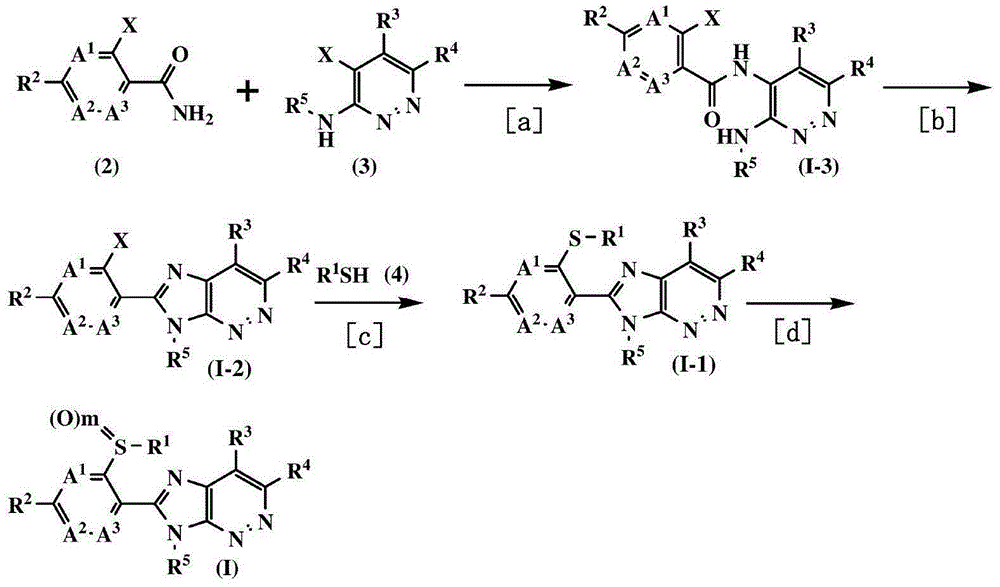
Fused heterocyclic compound or salt thereof, agricultural and horticultural insecticide containing fused heterocyclic compound, and method for using agricultural and horticultural insecticide
A kind of technology of condensed heterocyclic ring and compound, applied in the field of agricultural and horticultural pesticides, can solve the problems such as the condensed heterocyclic compound of undisclosed pyridazine ring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0648] Reference Example 1. Production of N-methyl-3-amino-6-trifluoromethylpyridazine
[0649]
[0650] A mixture of 6-trifluoromethyl-3-hydroxypyridazine (11.5 g), thionyl chloride (12.5 g), and dimethylformamide (1 ml) synthesized according to the method described in WO / 2005 / 047279 was heated to reflux for 3 Hour. Ice water was added to the reaction mixture, followed by extraction with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was dissolved in tetrahydrofuran (5 ml), and added dropwise to methylamine (40% methanol solution, 16.2 g) under ice-cooling. After stirring overnight at room temperature, water was poured into the reaction mixture, followed by extraction with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The obtained residue was subjected to column chromatography to obtain the target compound (3'-1) (5....
reference example 2
[0652] Reference example 2. Synthesis of N-methyl-3-amino-4-bromo-6-trifluoromethylpyridazine
[0653]
[0654] N-methyl-3-amino-6-trifluoromethylpyridazine (1.8 g) (3'-1), 3,5-dibromohydantoin (3.15 g) produced in Reference Example 1, The mixture of acetonitrile (10ml) was heated to reflux for 3 hours. A saturated sodium thiohydrogensulfate solution was poured into the reaction mixture, followed by extraction with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The obtained residue was subjected to column chromatography to obtain the target compound (3') (0.9 g).
[0655] Physical properties: 1 H-NMR (CDCl 3 ): 7.70 (s, 1H), 5,41 (brs, 1H), 3,26 (d, 1H)
manufacture Embodiment 1-1
[0657] Production of 2-(2-fluoro-4-trifluoromethylphenyl)-3-methyl-6-trifluoromethyl-3H-imidazo[4,5-C]pyridazine
[0658]
[0659] N-methyl-3-amino-4-bromo-6-trifluoromethylpyridazine (475 mg) (3′), 2-fluoro-4-trifluoromethylbenzamide ( A mixture of 500 mg) (2'), potassium tert-butoxide (311 mg), diphenylphosphinoferrocenepalladium dichloride (151 mg), toluene (5 ml) was heated at reflux under argon for 12 hours. Water was poured into the reaction mixture, followed by extraction with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The resulting residue was subjected to column chromatography to obtain the target compound (I'-2) (0.16 g).
[0660] Physical properties: 1 H-NMR (CDCl 3 ): 8.21(s, 1H), 7.97(t, 1H), 7.72(d, 1H), 7.64(d, 1H), 4.12(d, 3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com