A combination vaccine for inhibiting and/or preventing type a streptococcal infection
A streptococcus and vaccine technology, applied in the direction of antibacterial drugs, bacterial antigen components, etc., can solve the problems of inability to exert immune protection, hinder vaccine development, and restrict vaccine research and development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
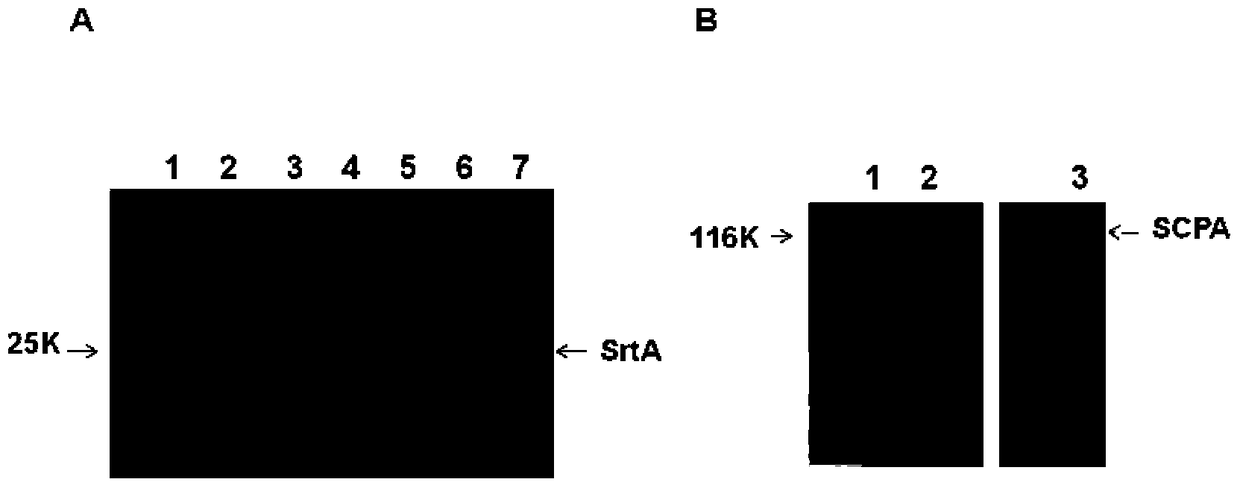
[0061] Embodiment 1, the preparation of combined vaccine component sortase A and C5a protease
[0062] 1. Preparation of sortase A
[0063] 1. Preparation of recombinant expression vector
[0064] 1) Using the genomic DNA of type A Streptococcus M1 as a template, perform PCR amplification with a primer pair composed of F1 and R1 to obtain a 516bp PCR amplification product, which is the sortase A gene SrtA.
[0065] F1: 5'-CTTA CATATG GTCTTGCAAGCACAAATGG-3';
[0066] R1: 5'-ATGTT CTCGAG CTAGGTAGATACTTGGTTATAAGA-3'.
[0067] 2), using restriction endonucleases NdeI and XhoI to double digest the PCR amplified product of step 1, and reclaim the digested product.
[0068] 3) The vector pET28a(+) was double digested with restriction endonucleases NdeI and XhoI, and the vector backbone of about 6000 bp was recovered.
[0069] 4) Ligate the digested product of step 2 with the vector backbone of step 3 to obtain the recombinant plasmid pET28a-SrtA.
[0070] After sequencing, t...
Embodiment 2
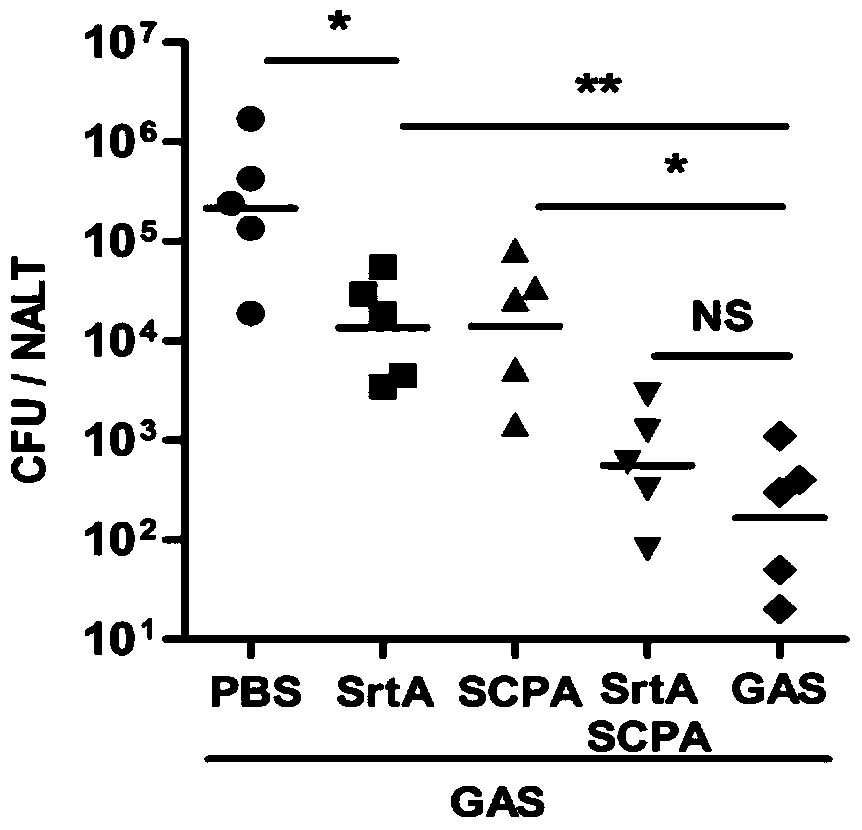
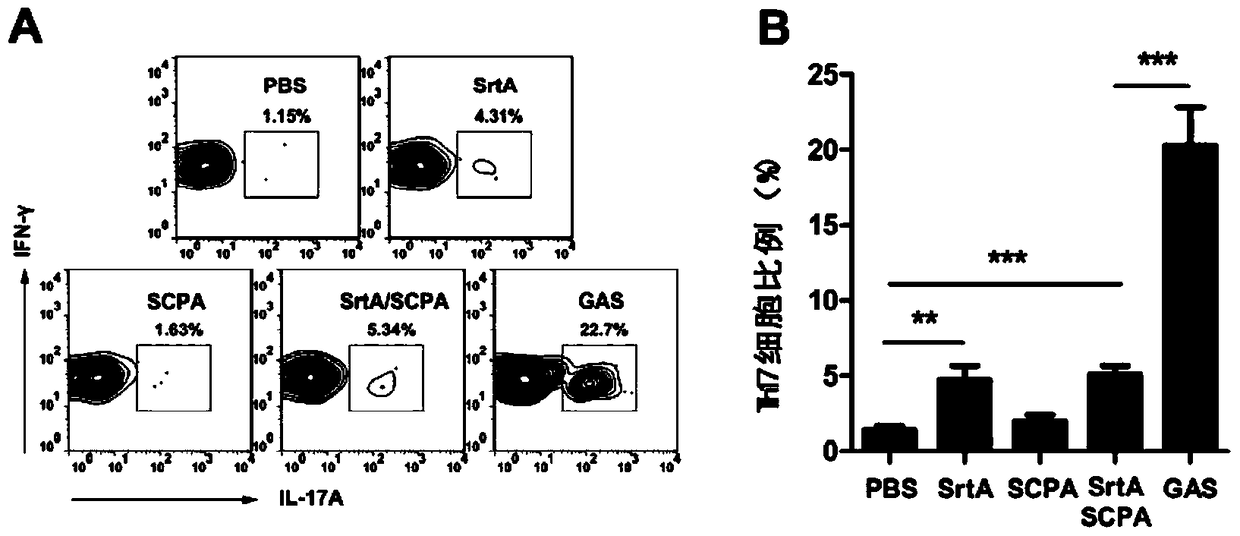
[0109] Embodiment 2, the functional research of combined vaccine
[0110] 1. Immune protection of combined vaccine against Strep A bacteria
[0111] The immunogens are grouped as follows:
[0112] Sortase A plus CTB group (SrtA): the sortase A prepared in Example 1 and the adjuvant CTB were mixed at a mass ratio of 10:1 for immunization, and the immunization dose was 10 μg sortase A / time / body;
[0113] C5a protein plus CTB group (SCPA): the C5a protease prepared in Example 1 and the adjuvant CTB were mixed according to the mass ratio of 20:1, and immunized, and the immunization dose was 20 μg C5a protein / time;
[0114] Combined vaccine plus CTB group (SrtA / SCPA): Combined vaccine immunization prepared in Example 1, immunization dose is 20 μg C5a protein / time;
[0115] Streptococcus A full bacteria group (GAS): Type A Streptococcus M1 type immunity, the immune dose is 5×10 7 cfu / time;
[0116] PBS group: use PBS (10mM Na 2 HPO 4 , 1.8mM KH 2 PO 4 , 140mM NaCl, 2.7mM KCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com