Method for preparing hydroxy neovaleraldehyde by condensing formaldehyde and isobutyraldehyde
A technology for hydroxypivalaldehyde and isobutyraldehyde is applied in the field of preparing hydroxypivalaldehyde and can solve the problems of low conversion rate and selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
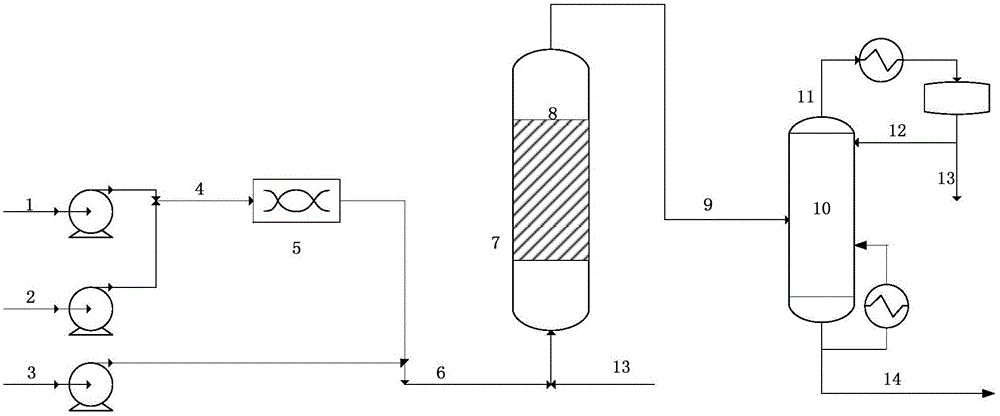
[0025] in such as figure 1 In the shown technological process, formaldehyde and isobutyraldehyde mol ratio are 1.05:1, and the formaldehyde aqueous solution (formaldehyde mass content is 37wt%) of about 432g / h feed rate, about 372g / h industrial isobutyraldehyde (isobutyraldehyde Mass content > 98wt%), the two are put into the stirred tank for stirring and mixing, the outlet material and the trimethylamine catalyst with a flow rate of about 72g / h are added to the tubular reactor equipped with quartz sand, and the material direction is from bottom to top. At a reaction temperature of 75°C, a pressure of normal pressure, and a residence time of 1 hour, the reaction generates an aqueous solution of hydroxypivalaldehyde, and the composition of the outlet material is about 59wt% hydroxypivalaldehyde, 1.7wt% isobutyraldehyde, and 1.43wt% formaldehyde , 0.7wt% trimethylamine, 0.8wt% 1115 ester, the balance is water. The reaction results are: the conversion rate of isobutyraldehyde is...
Embodiment 2
[0027] in such as figure 1 In the shown technological process, formaldehyde and isobutyraldehyde mol ratio are 1.1:1, and the formaldehyde aqueous solution (formaldehyde mass content is 37wt%) of about 450g / h feed rate, about 372g / h industrial isobutyraldehyde (isobutyraldehyde Mass content > 98wt%)), the two are added into a stirred tank for stirring and mixing, and the outlet material is passed into a tubular reactor filled with 105g of basic anion exchange resin 201-7, and the material direction is from bottom to top. Under the conditions of reaction temperature 75°C, normal pressure, and residence time 1h, the reaction produces an aqueous solution of hydroxypivalaldehyde. The composition of the outlet material was about 57.93 wt% hydroxypivalaldehyde, 2.33 wt% isobutyraldehyde, 4.3 wt% formaldehyde, 1.45 wt% 1115 ester, and the balance was water. The reaction results are: the conversion rate of isobutyraldehyde is 96.57%, and the selectivity of hydroxypivalaldehyde+neopen...
Embodiment 3
[0029]According to the conditions and steps described in Example 1, 700ml of θ ring metal bulk packing (porosity about 0.85) is loaded into the tubular reactor, and the reaction temperature is 75°C, the pressure is normal pressure, and the residence time is 1h. Aqueous solution of hydroxypivalaldehyde. The reaction result is: the conversion rate of isobutyraldehyde is 95%, and the selectivity of hydroxypivalaldehyde+neopentyl glycol is 97.53%. The condensation product enters the vacuum rectification tower for separation, reclaims unreacted formaldehyde isobutyraldehyde and catalyst, and the conditions and steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com