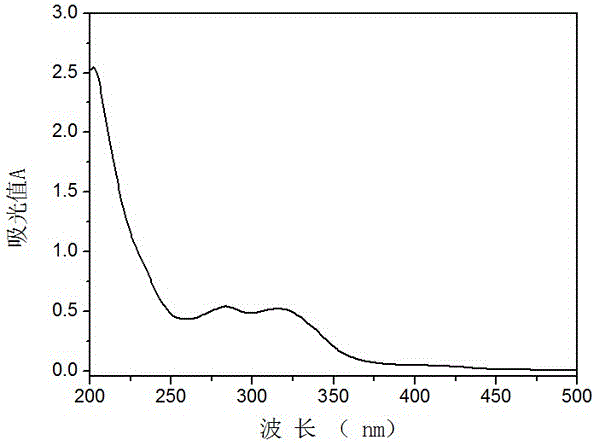
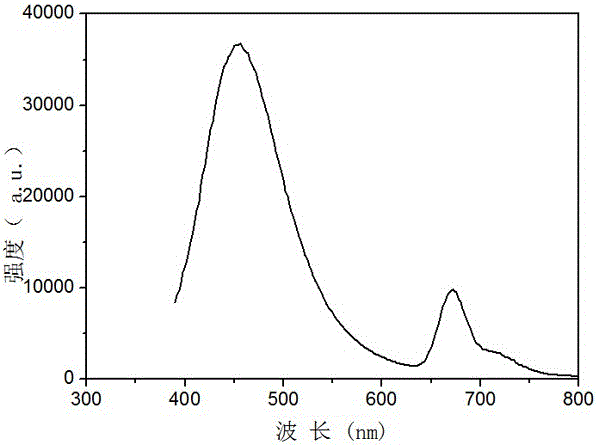
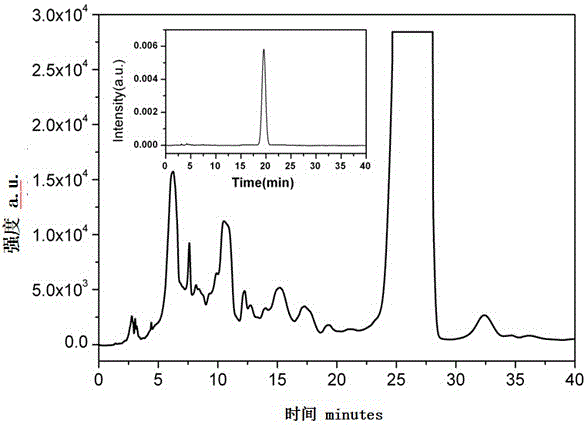
Hexamethoxyflavanone-rhamnosyl-rhamnoside and application thereof
A technology of hexamethoxyflavanone and rhamnosyl, applied in the field of hexamethoxyflavanone-rhamnosyl-rhamnoside and its application, can solve the problem that chemotherapy drugs cannot prevent tumor metastasis and reduce cancer death rate, attack the patient's immune system and other problems, and achieve obvious anti-tumor cell migration and invasion ability, and promote the effect of tumor cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation liquid phase separation conditions in the step (4) are: chromatographic column: AgelaVenusilXBPC18; flow rate: 1-4ml / min; column temperature: 35°C; detection fluorescence excitation wavelength 352nm, emission wavelength 458nm, isocratic elution, The mobile phase is a mixture of methanol and pure water, and the volume ratio of methanol and pure water is 9:11. The injection volume is 200μL, and the 20mg / mL sample is prepared with methanol solvent.
[0038] The operating conditions of the mass spectrometer in the step (4) are: ion source: atmospheric pressure electrospray ion source (ESI); scan mode: full scan in positive ion mode; scan range: 150-800 m / z ; spray voltage (spray voltage): 4.0kV; sheath gas flow rate (sheath gas flow rate): 35 Arb; auxiliary gas flow rate (aux gas flow rate): 5 Arb; ion transfer tube temperature (capillary temperature): 350 ° C; tube diameter voltage (tubelens): 60.00V.
Embodiment 1 9
[0039] The extraction method of the active ingredient of embodiment 1 Murata japonica
[0040] Take 4.2Kg of dried Jiulixiang medicinal materials (twigs) and crush them, and reflux extract them in 5L of 75% ethanol. Each batch of medicinal materials is extracted 3 times for 3 hours each time. Combine and reflux the extracts, concentrate the ethanol under reduced pressure to obtain a syrupy total extract. The total extract was ultrasonically suspended in water, the suspension was extracted with dichloromethane, the extraction ratio was 1:1 (v:v), extracted 4 times, combined and concentrated under reduced pressure, the obtained crude extract was placed on a silica gel column, distilled with ethyl acetate -Petroleum ether was separated by gradient chromatography, the fraction eluted with ethyl acetate was collected, and the solvent was removed by rotary evaporation to obtain the target component with a yield of about 0.09%. The extract was dissolved in an equivalent amount of me...
Embodiment 2
[0041] Embodiment 2 semi-preparative chromatography
[0042] Conditions are, instrument: Waters2695; detector: Waters2475FLR; chromatographic column: AgelaVenusilXBPC18 (10mm×250mm, 5μm); flow rate: 3mL / min; column temperature: 35°C; detection wavelength: λex=352nm, λem=458nm; isocratic Elution and methanol and pure water according to the ratio of 9:11. Inject 200μl of 20mg / mL sample, collect the components of 23-27min, such as image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com