Nogo-A receptor binding peptide as well as derivative and application thereof
A receptor binding and derivative technology, applied in the field of biomedicine, can solve the problems of nerve function recovery of patients, achieve huge social and economic benefits, promote recovery, and promote nerve regeneration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
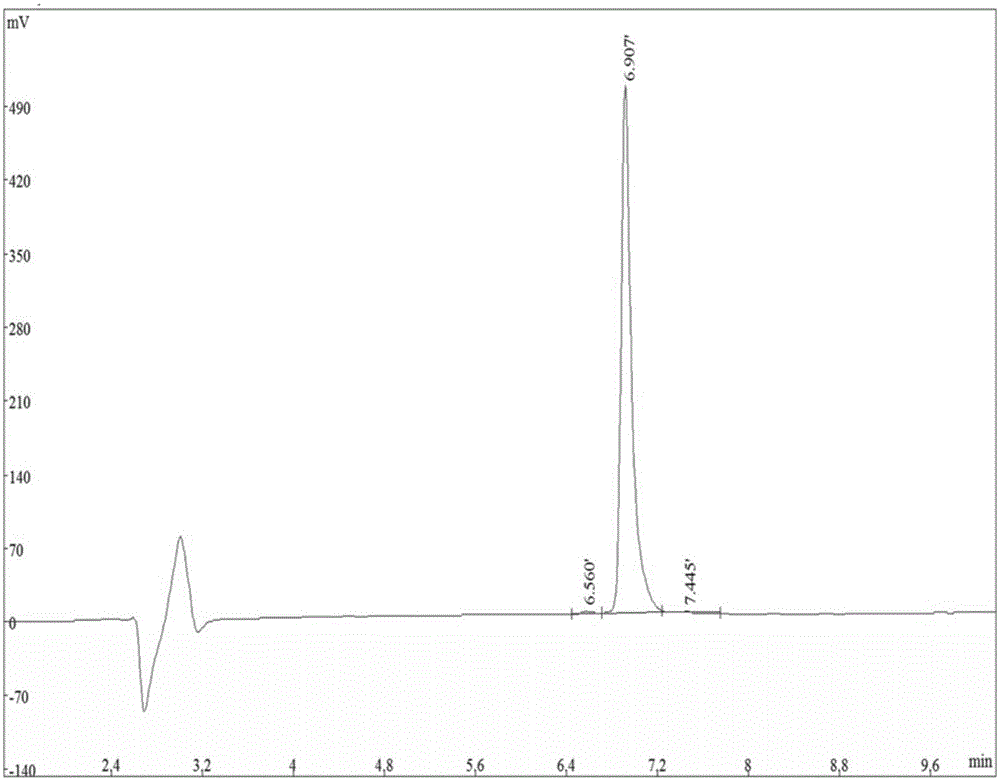
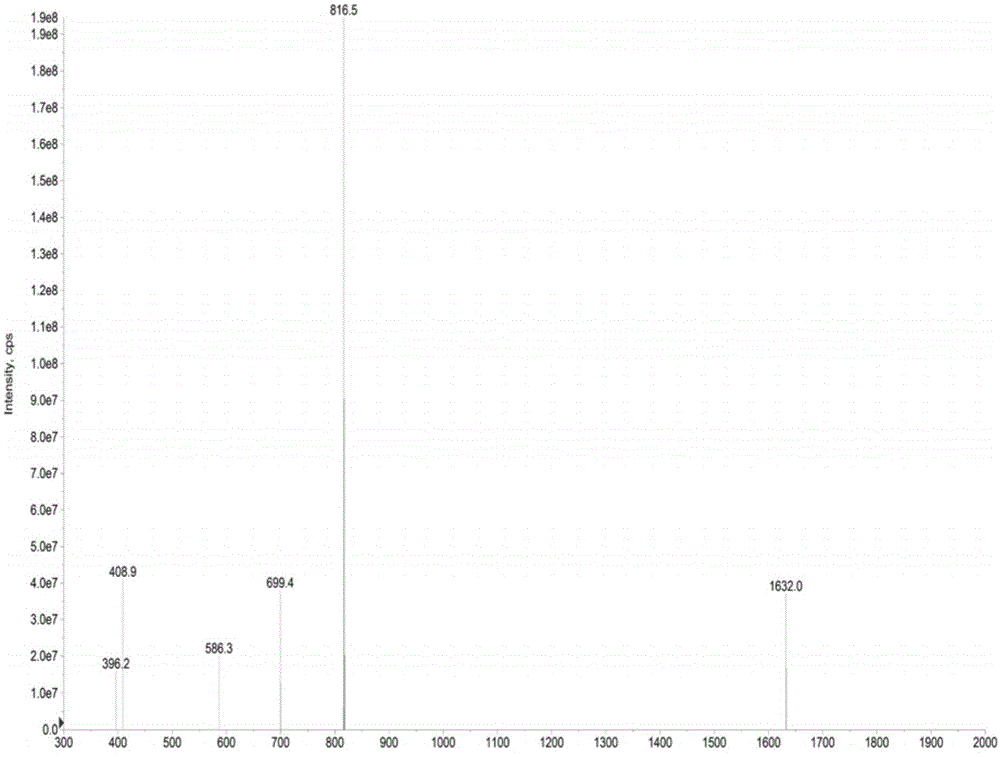
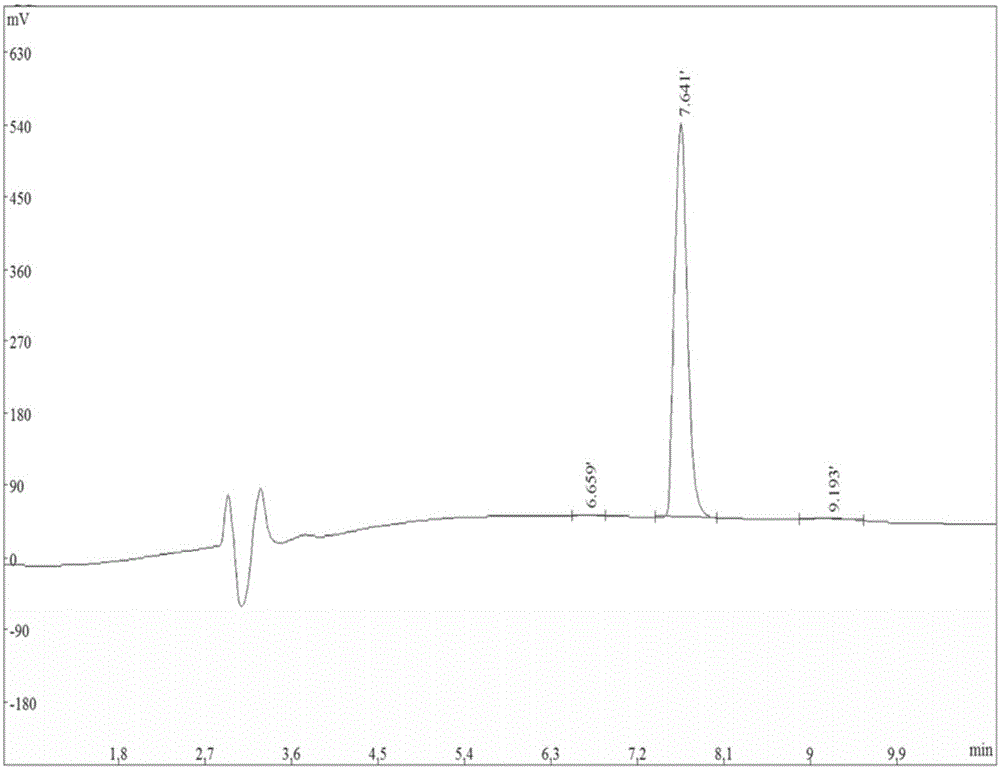
[0047] Example 1 Organic Synthesis and Detection of Nogo-A Receptor Binding Peptides
[0048] Organically synthesized No. 2 polypeptide (His-Ile-Tyr-Thr-Ala-Leu-Val) and No. 5 polypeptide (Gly-Phe-Ala-Thr-Ile-Thr-Gly) (synthesized by Shanghai Qiangyao Biological Company), called Take 1g of KP resin and place it in a reactor, add DMF to swell for 30min, filter out DMF, dissolve Fmoc amino acid, 0.4mmol of HBTU, HOBT and 0.6mmol of NMM in an appropriate amount of DMF, add it to the reactor, and set it at 40°C Shake for 2 hours, filter the reaction solution, wash the resin twice with methanol and DMF alternately, and wash once with anhydrous ether; elute the Fmoc group with 20% piperidine / DMF solution by volume fraction; react for 10 minutes, filter the reaction solution methanol, Wash the resin twice with DMF alternately; carry out the coupling of the next amino acid residue, so that the first amino acid at the C-terminal is extended to the N-terminal until the short peptide is ...
Embodiment 2
[0050] Example 2 ELISA detection of phage display: NgR specificity, specificity binding positive monoclonal
[0051] A 96-well ELISA plate (Corning, Inc.) was coated with sNgR protein diluted in PBS at a concentration of 2.5 μg / mL (R&D Company, USA), 150 μL per well, and the wells not coated with sNgR were used as a control. Shake slowly overnight at 4°C, pour off the coating solution, add a blocking solution with a mass fraction of 1% BSA diluted in PBS, and block for 1 hour; add the monoclonal 1×10 10100 μL of pfu / mL phage (NewEngland Biolabs, Beverly, MA), combined with slow shaking at room temperature for 1 h; then washed 6 times with 0.1% PBST, and added 100 μL of HRP-anti-M13 antibody (NEB Company) (diluted 1:5000) , incubate at 37°C for 40 min, wash with PBST for 6 times, add 50 μL of the display substrate TMB (Beiyuntian Company) for 10 min, 1MH 2 SO 4 Stop the color development, and detect the OD value with a microplate reader at 450nm. According to the results of ...
Embodiment 3
[0052] Example 3 Confocal binding of Nogo-A receptor-binding peptides to NgR on the cell membrane surface of PC-12 and CGCs cells with high NgR expression, and colocalization of Nogo-A receptor-binding peptides with NgR on the surface of PC-12 cells
[0053] Confocal plates were inoculated with PC-12 and CGCs cells, among which PC12 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (ATCCCRL-1721), and CGCs cells were obtained from Sprague-Dawley (SD) suckling mice (SPF grade, Guangdong Province, 6-8 days after birth) Center (SCXK Guangdong 2013-0002)); 10,000 cells per dish, cultured for 24 hours, washed three times with PBS, fixed with paraformaldehyde for 15 minutes, washed three times with PBS, blocked with BSA for 1.5 hours, and then labeled with 1 μM FITC peptide No. 2 Or polypeptide No. 5 (the FITC markers of polypeptide No. 2 or No. 5 were prepared by Shanghai Qiangyao Biological Co., Ltd.), incubate at room temperature for 2 hours (co-localizati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com