A class of amylase mutants with improved thermostability and their coding genes and applications
A technology encoding gene and amylase, which is applied in application, genetic engineering, plant gene improvement, etc., can solve the problems of poor thermal stability and achieve excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
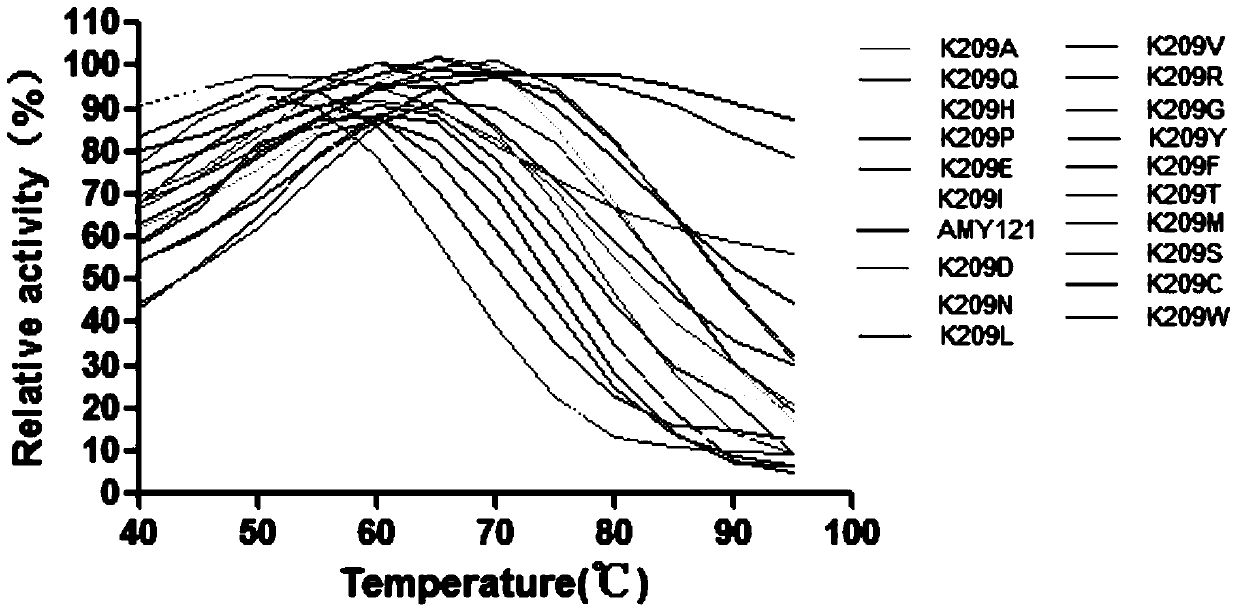
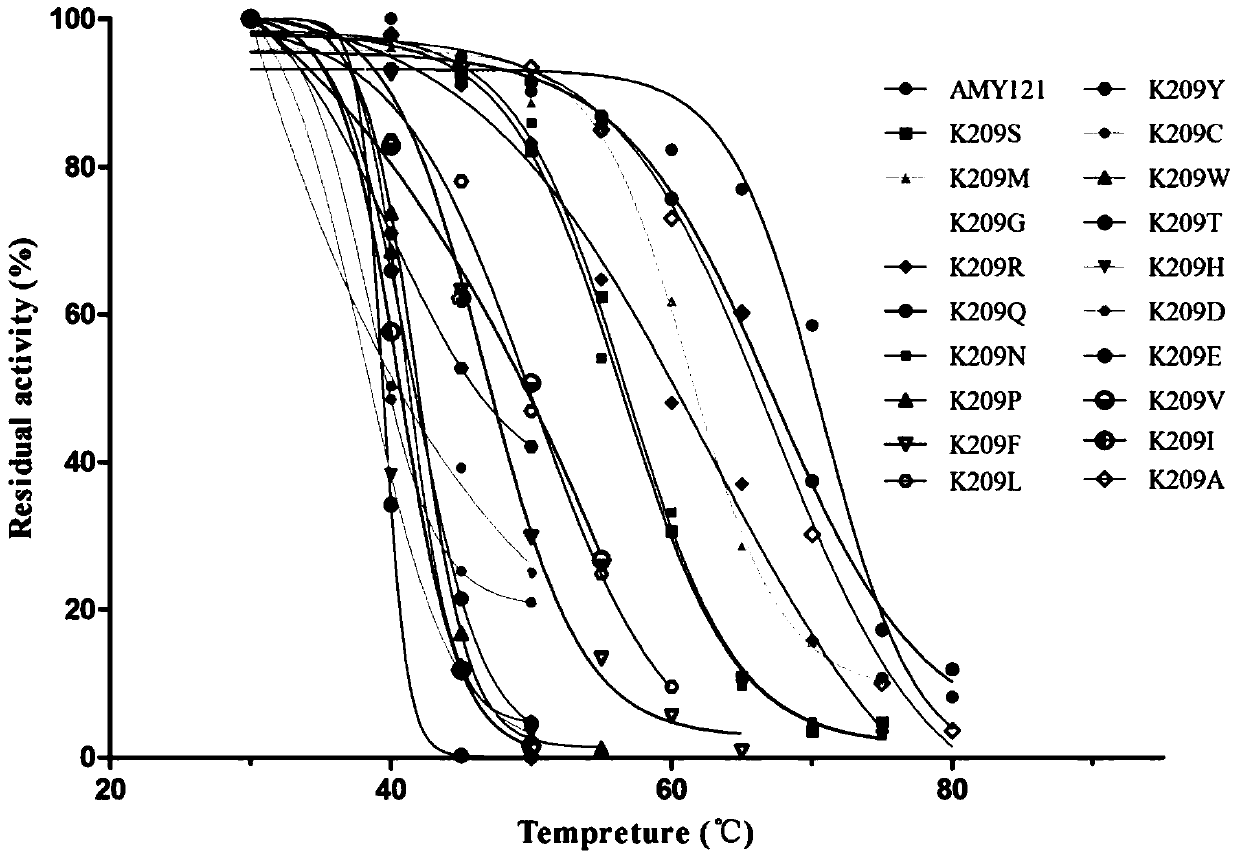
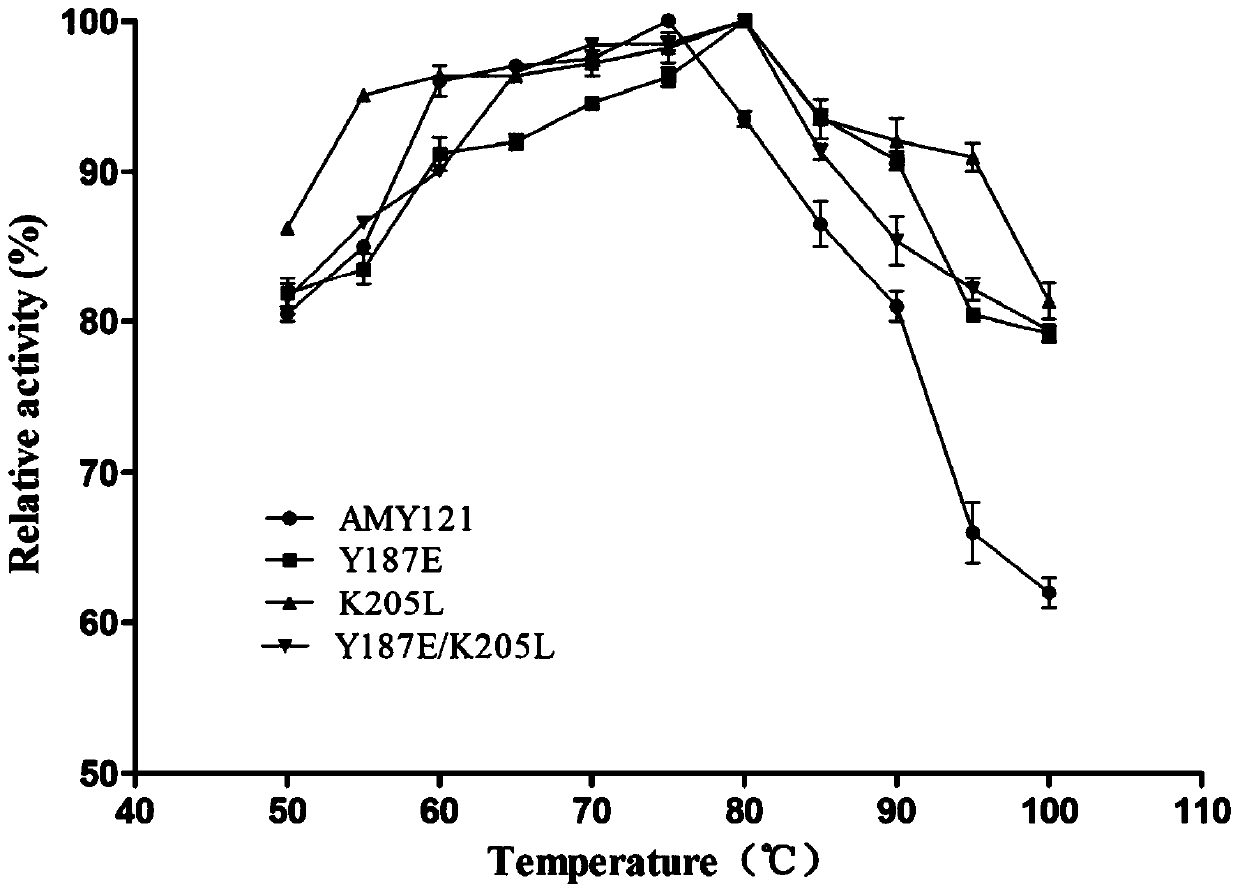
[0025] After inserting two amino acids between the 208th and 209th amino acid residues of α-amylase AMY121 in the thermostable bacterium Bacillus sp.SCSIO 15121 from deep sea vents, its thermal stability decreased significantly. Through homology modeling, it was found that the insertion of two amino acids significantly changed the conformation of Lys209. The amino acid sequence of the α-amylase AMY121 is shown in SEQID NO.1, and the nucleotide sequence of its coding gene—α-amylase gene amy121 has been deposited in Genbank, and its accession number is KJ577547.
[0026] 1 Identification of amino acid residue Lys209 related to thermostability
[0027] 1.1 Site-directed mutation of Lys at position 209 of α-amylase AMY121 mediated by inverse PCR
[0028] In order to further study the amino acid residue Lys209 related to the thermostability of α-amylase AMY121, starting from the deep sea vent bacterium Bacillus sp. Primers for saturation mutations, using inverse PCR to mediate si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com