Combination containing M10C1 compound and active ingredient and preparation method of combination
A technology of M10C1 and active ingredients, applied in the field of compositions comprising M10C1 compounds and active ingredients and their preparation fields, can solve the problems of short half-life, difficult to pass through cell membranes, produce strong inflammatory response, etc., to achieve changes in biodistribution, improve Pharmacokinetic characteristics, effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
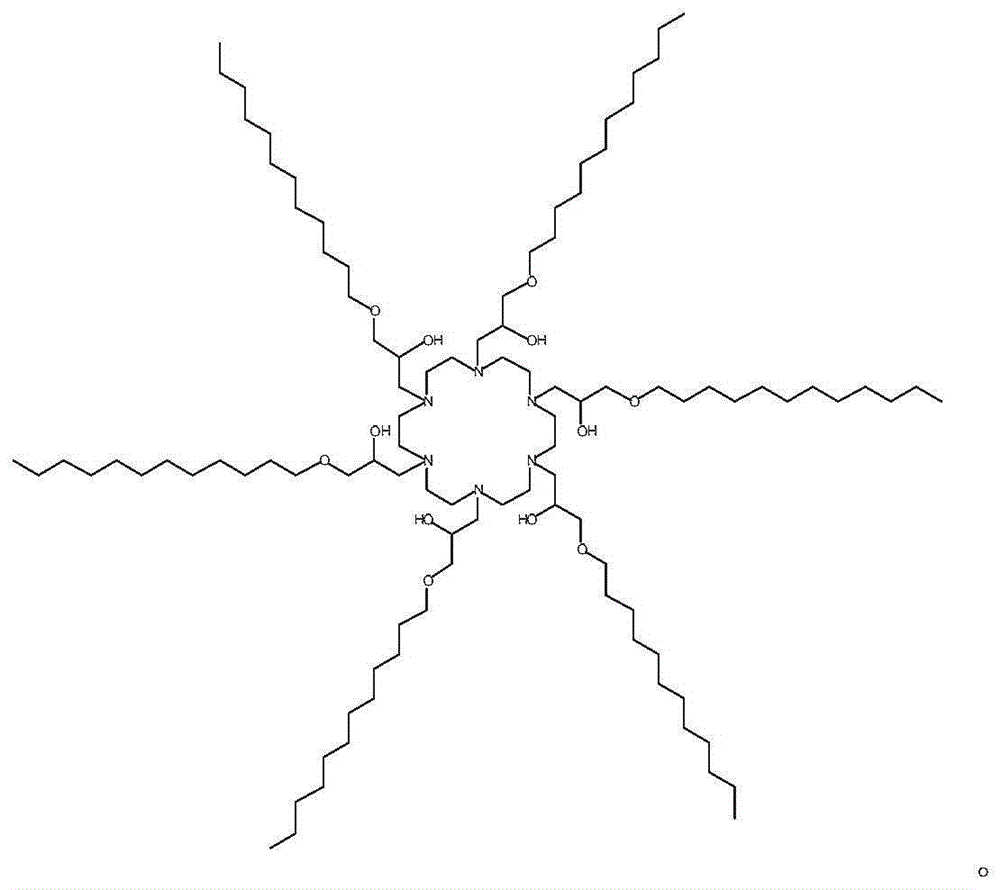
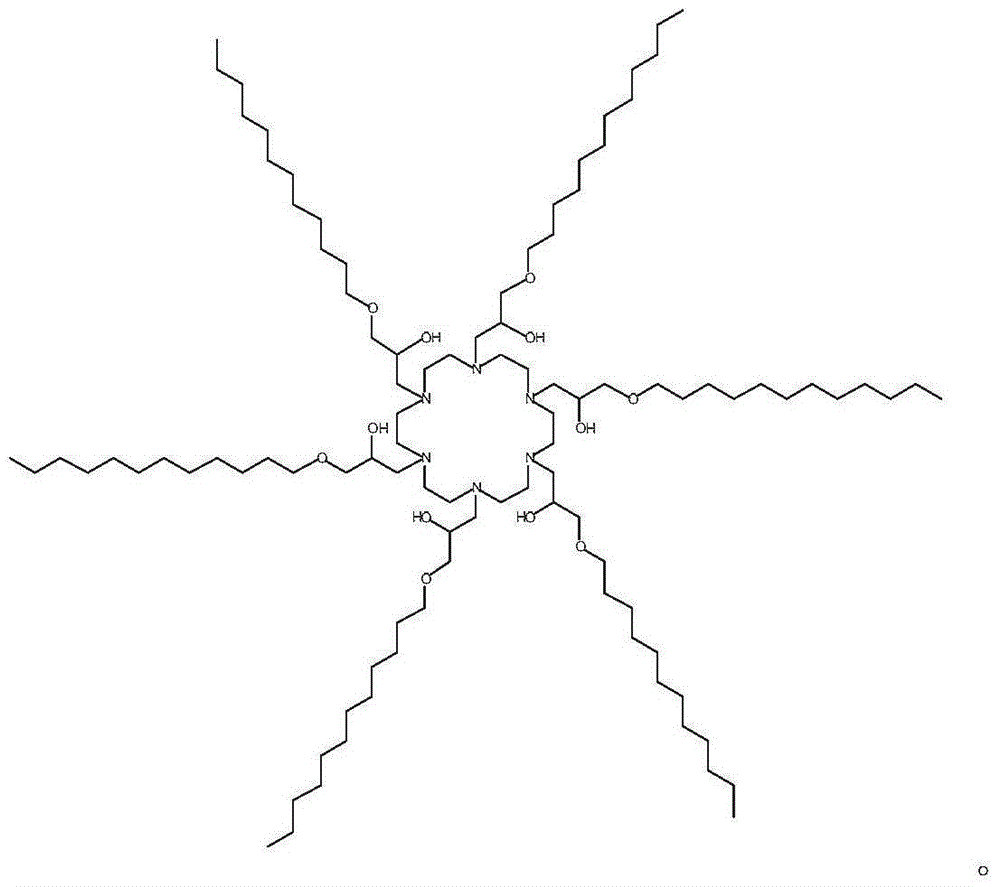
[0026] Embodiment 1 synthetic compound M10C1 compound
[0027] The M10C1 compound is obtained by reacting (1,4,7,10,13,16-hexaazacycloctadecane hexahydrochloride) and an epoxy compound at 90° C. without solvent in a glass bottle equipped with a stirring bar. The reaction time is 24-72 hours at 90°C. Its N, N-bis (3-aminopropyl) methylamine and epoxy alkyl compound (alkyl (C12-C14) glycidyl ether, C12-14 glycidyl ether ratio (molar ratio) in the reaction is 1: 4. Its result is known to obtain confirmation from thin-layer chromatography (TLC), and there is only a kind of main product in the reaction mixture.The product of reactant can be applied after being purified;
Embodiment 2
[0029] The synthetic method of cholesterol-polyethylene glycol compound is: place in 2000 milliliters of round bottom flasks, be (1S, 2R, 5S, 10S, 11S, 14R, 15R)-2,15-dimethyl-14-[(2R )-6-methylheptyl-2 base]tetracyclo[8.7.0.0^[2,7].O^[11,15]]heptadecan-7-en-5-ol (200 grams, 517.26mmol , 1.0 equivalents, pyridine (1000 milliliters). Add 4-toluenesulfonyl chloride (148 grams, 776.30 1.50 equivalents). Stir overnight in an oil bath at 50° C. The reaction mixture is cooled to a cryogenic room with water / ice bath. Add 2000 milliliters of ether to dilute .To the mixture was added water 1×1000 water and 5×1000 20% citric acid. The resulting mixture was washed with 1×1000 ml sat. , 11S, 14R, 15R)-2,15-dimethyl-14-[(2R)-6-methylheptyl-2-yl]tetracyclo[8.7.0.0^[2,7].0^[ 11,15]] Heptadecan-7-en-5-yl 4-methylphenyl-1-sulfonate was placed in a 3000 ml round bottom flask (1S, 2R, 5S, 10S, 11S, 14R, 15R )-2,15-dimethyl-14-[(2R)-6-methylheptyl-2-yl] tetracyclo [8.7.0.0^[2,7].0^[11,15]] ten ...
Embodiment 3
[0031] Carriers for siRNA drugs:
[0032] Justice chain: CCGUGUGCACUUCGCUUCA[dT][dT];
[0033] Antisense strand: UGAAGCGAAGUGCACACGGUC.
[0034] The RNA interference composition also includes components in the following weight ratios:
[0035] The M10C1 compound 1g that embodiment 1 makes
[0036] Cholesterol-polyethylene glycol 10002g;
[0037] The above-mentioned preparation method of the RNA interference composition for the treatment of hepatitis B comprises the following steps:
[0038] (1) dissolving one of the siRNA sequence groups in 0.9% water and 5% sucrose or pure water to obtain solution 1;
[0039] (2) Take the following components by weight, and dissolve these components in alcohol to obtain solution two:
[0040] M10C1 compound 1g
[0041] Cholesterol-polyethylene glycol 10002g;
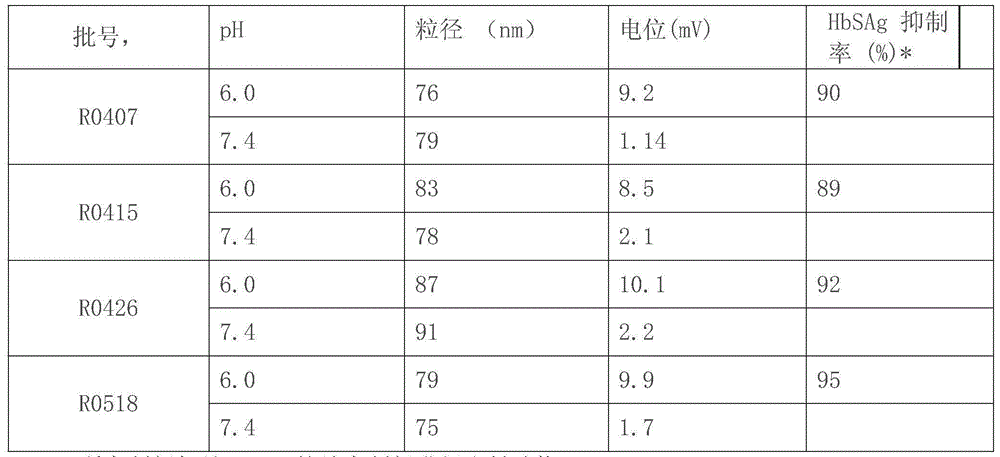
[0042] The weight ratio of the weight sum of the components in step (2) to one group or mixture in the siRNA sequence group is 2-10:1; the volume ratio of solution one to solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com