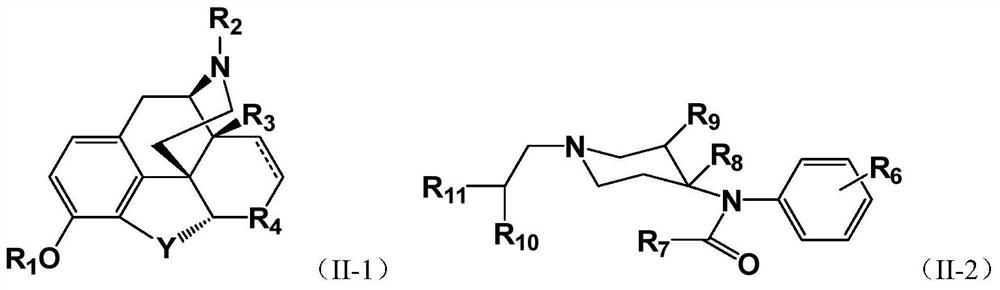
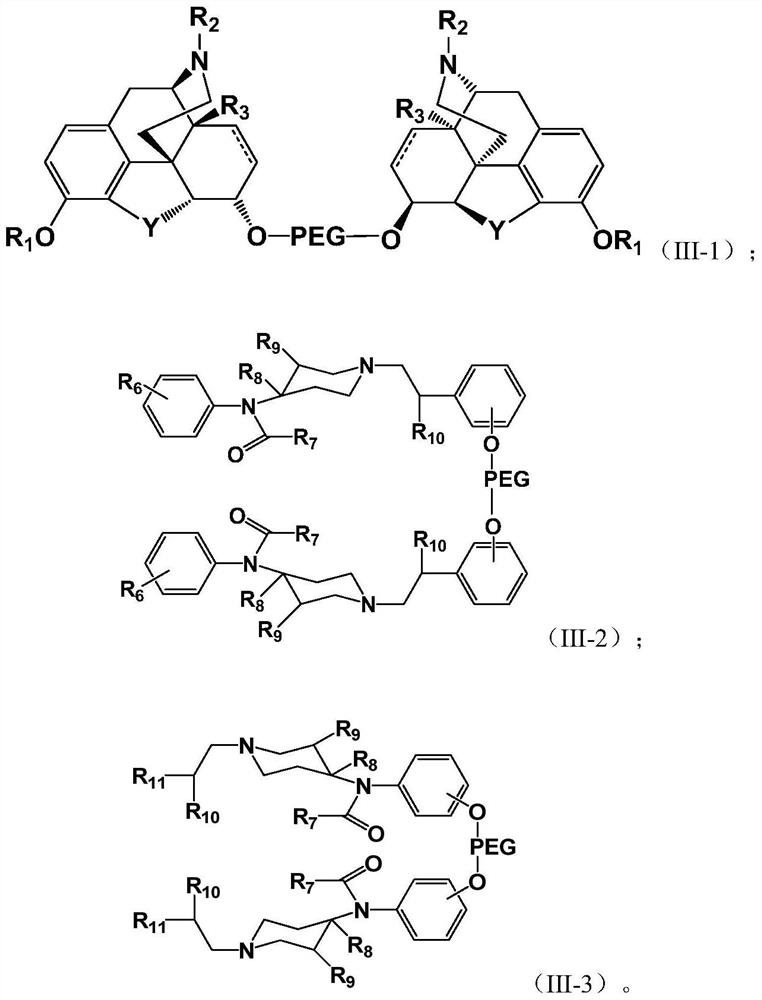
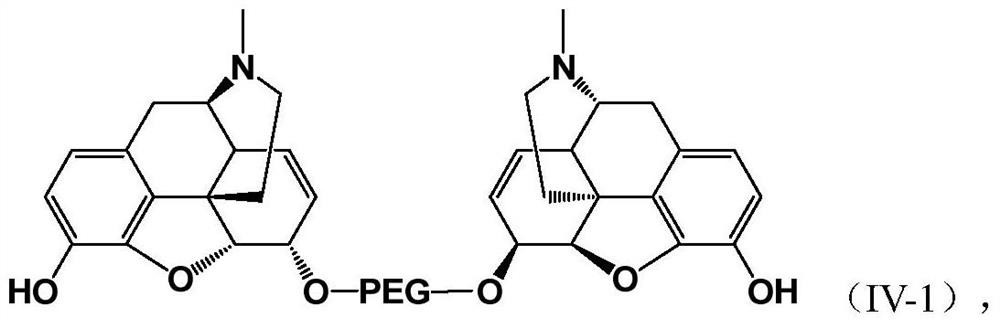
PEGylated Opioids with Low Addictive Effects
An opioid, polyethylene glycol technology, applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of reducing drug addiction, opioid and opium Problems such as decreased receptor affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Embodiment 1: prepare PEG n -(OMs) 2
[0111] Add diethylene glycol (10.0 g, 94.3 mmol) and dichloromethane (100 mL) into a 250 mL three-necked flask, stir and cool to 0°C. Triethylamine (19.1 g, 188.6 mmol) was added dropwise, and the reaction mixture was stirred for 10 minutes. Methanesulfonyl chloride (21.6 g, 188.6 mmol) was added to the reaction mixture over 5-10 minutes, stirred at room temperature for 12 hours, and the progress of the reaction was monitored by TLC. After the reaction was complete, distilled water was added to the reaction mixture, followed by extraction with dichloromethane (3×100 mL). The organic layers were combined, washed with distilled water (3×100 mL), dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure with a rotary evaporator to obtain 21.8 g of a brown oil with a yield of 88.2%. 1 H NMR (400MHz, CDCl 3 ): δ4.10(t,4H), 3.86(t,4H), 3.19(s,6H).
[0112] Prepare other MsO-PEG in the same way n -OMs ...
Embodiment 2
[0113] Embodiment 2: Preparation of PEG n -(morphine) 2 Conjugate
[0114]
[0115] Step 1: Preparation of 3-O-MEM-morphine (2)
[0116] Morphine sulfate (1.00 g, 2.64 mmol) was dissolved in acetone / toluene (70 mL / 35 mL) at room temperature, and the mixture was stirred homogeneously. Add K to the mixture 2 CO 3 (1.35g, 9.77mmol), stirred for 25 minutes, then added MEMCl (0.66g, 5.28mmol). The reaction mixture was stirred at room temperature for 24 hours, then quenched by the addition of anhydrous methanol (1.2 mL). The reaction mixture was concentrated to dryness under reduced pressure, distilled water (15 mL) and saturated brine (45 mL) were added to the residue, and extracted with ethyl acetate (3×50 mL). The combined organic solution was washed with saturated brine (3×50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by Biotage flash purification preparative liquid chromatography to obtain 0.52 g of a l...
Embodiment 3
[0121] Embodiment 3: preparation PEG n -(codeine) 2 Conjugate
[0122]
[0123] Step 1: Preparation of 6-O-PEG n -(codeine) 2 Free base (6)
[0124] Add codeine (2.4-2.8 equivalents) to a mixed solution of toluene / DMF (24 times / 1 times volume), followed by NaH (8-12 times equivalents), and then PEG n -(OMs) 2 . The reaction mixture was heated to 45-65° C. and kept stirring until the completion of the reaction was confirmed by LC-MS analysis (12-48 hours depending on PEG chain length). The reaction was terminated with anhydrous methanol (10 volumes), and the reaction mixture was concentrated to dryness under reduced pressure. The residue was purified by Biotage flash preparative liquid chromatography to obtain a yellow to orange oil with a yield of 12 to 25%. 6-O-PEG was prepared by this method n -(codeine) 2 Free base (n=1, 2, 3, 4, 5, 6, 7, 8, 9), the product is 1 Confirmed by H NMR and LC-MS.
[0125] Step 2: Preparation of 6-O-PEG n -(codeine) 2 Hydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com