Application of cistanche deserticola polysaccharides as vaccine adjuvant
A technology of Cistanche deserticola polysaccharide and vaccine adjuvant, applied in the field of immune adjuvant, can solve the problems of side effects, induced antibody response, etc., and achieve the effects of small side effects, simple preparation process and wide sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
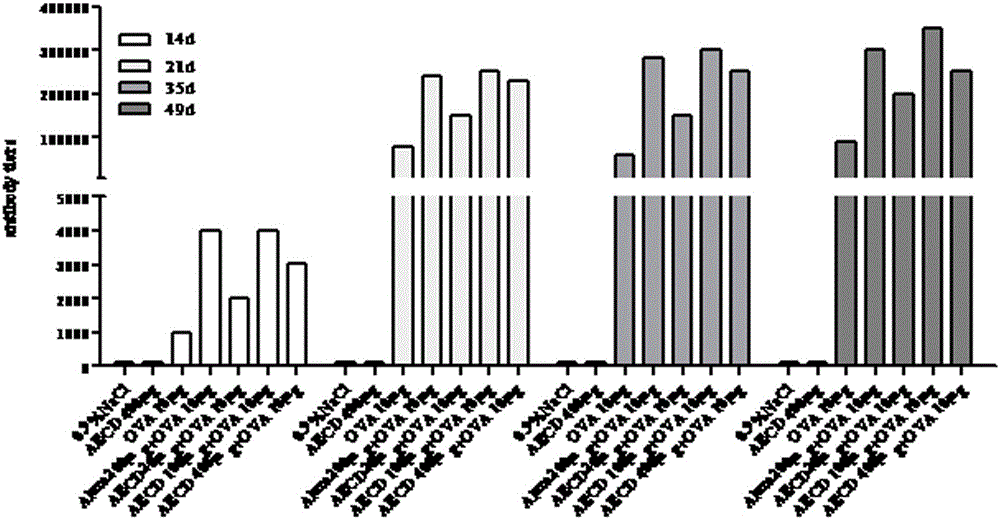
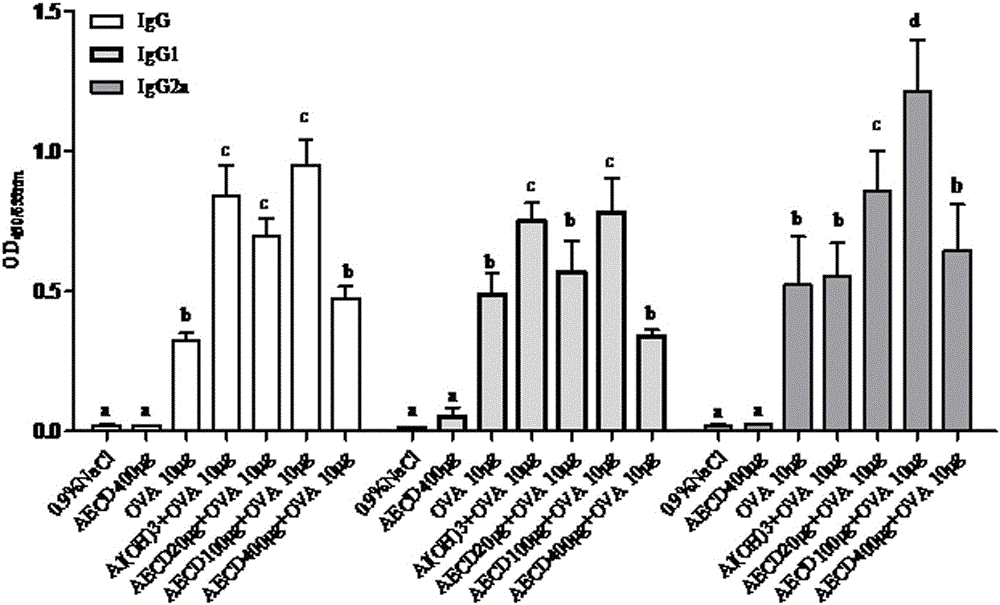
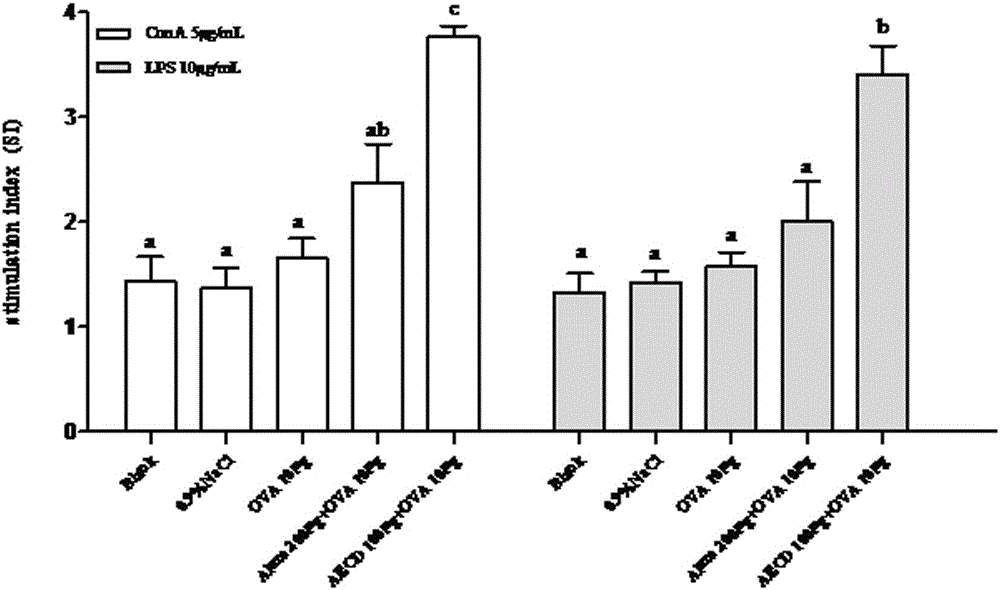
[0025] Example 1: Animal experiment of different doses of Cistanche deserticola polysaccharides on mouse immune model antigen ovalbumin (OVA)
[0026] The preparation method of present embodiment Cistanche deserticola polysaccharide is:
[0027] Step 1: Degrease fresh or dried Xinjiang Cistanche powder with petroleum ether, add distilled water for ultrasonication, add absolute ethanol to stir and precipitate the filtrate after concentration, centrifuge the precipitate, and vacuum dry to obtain the crude product of Cistanche deserticola polysaccharide;
[0028] Step 2: Use Sevage reagent (chloroform:n-butanol ratio: 4:1) to remove protein from cistanche cistanche, centrifuge, combine the supernatant, repeat the above operation several times, collect the supernatant, add ethanol, let stand overnight, and centrifuge the precipitate , and vacuum-dried to obtain the polysaccharide of Cistanche deserticola.
Embodiment approach
[0030] Test material: Cistanche polysaccharide mother liquor Configuration: 0.1g of Cistanche deserticola polysaccharide is dissolved in 10ml of normal saline to prepare a 10mg / ml mother liquor. Prepare OVA mother solution: dissolve 0.01g OVA in 10ml of normal saline, and prepare a 1mg / ml mother solution.
[0031] Animal immunization: ICR mice aged 6-8 weeks were randomly assigned to 7 groups, 6 mice in each group, and the experimental groups were shown in Table 1.
[0032] Table 1 Immune grouping of mice
[0033] Group No species (female) only count Injectable ingredients 1 ICR 6 0.9%NaCl 2 ICR 6 AECD 400 μg 3
6 OVA 10 μg 4 ICR 6 Alum200 μg+OVA 10 μg 5 ICR 6 AECD20μg+OVA 10μg (low dose) 6 ICR 6 AECD 100 μg+OVA 10 μg (medium dose) 7 ICR 6 AECD 400 μg+OVA 10 μg (high dose)
[0034] Each group of vaccines was dissolved in normal saline, and each mouse was injected with 100 microliters, subcutan...
Embodiment 2
[0053] Example 2: Cistanche deserticola polysaccharide adjuvant to immune model animal experiment of influenza virus inactivated vaccine (Flu)
[0054] The preparation of Cistanche deserticola polysaccharides in this example was prepared according to the method described in Example 1 of the present invention.
[0055] Test material: Cistanche cistanche multipond was prepared as in Example 1, and the influenza vaccine was commercially available.
[0056] Animal immunization: female ICR mice aged 6-8 weeks were divided into 6 groups, 6 mice in each group, and the experimental groups were shown in Table 6.
[0057] Table 6 Mouse immune groups
[0058] Group No species (female) only count Injectable ingredients 1 ICR 6 0.9%NaCl 2 ICR 6 AECD800μg 3 ICR 6 Influenza vaccine 0.5μg 4 ICR 6 Influenza vaccine 0.5μg+AECD100μg 5 ICR 6 Influenza vaccine 0.5μg+AECD400μg 6 ICR 6 Influenza vaccine 0.5μg+AECD800μg
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com